| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1305499 | 1499161 | 2015 | 6 صفحه PDF | دانلود رایگان |
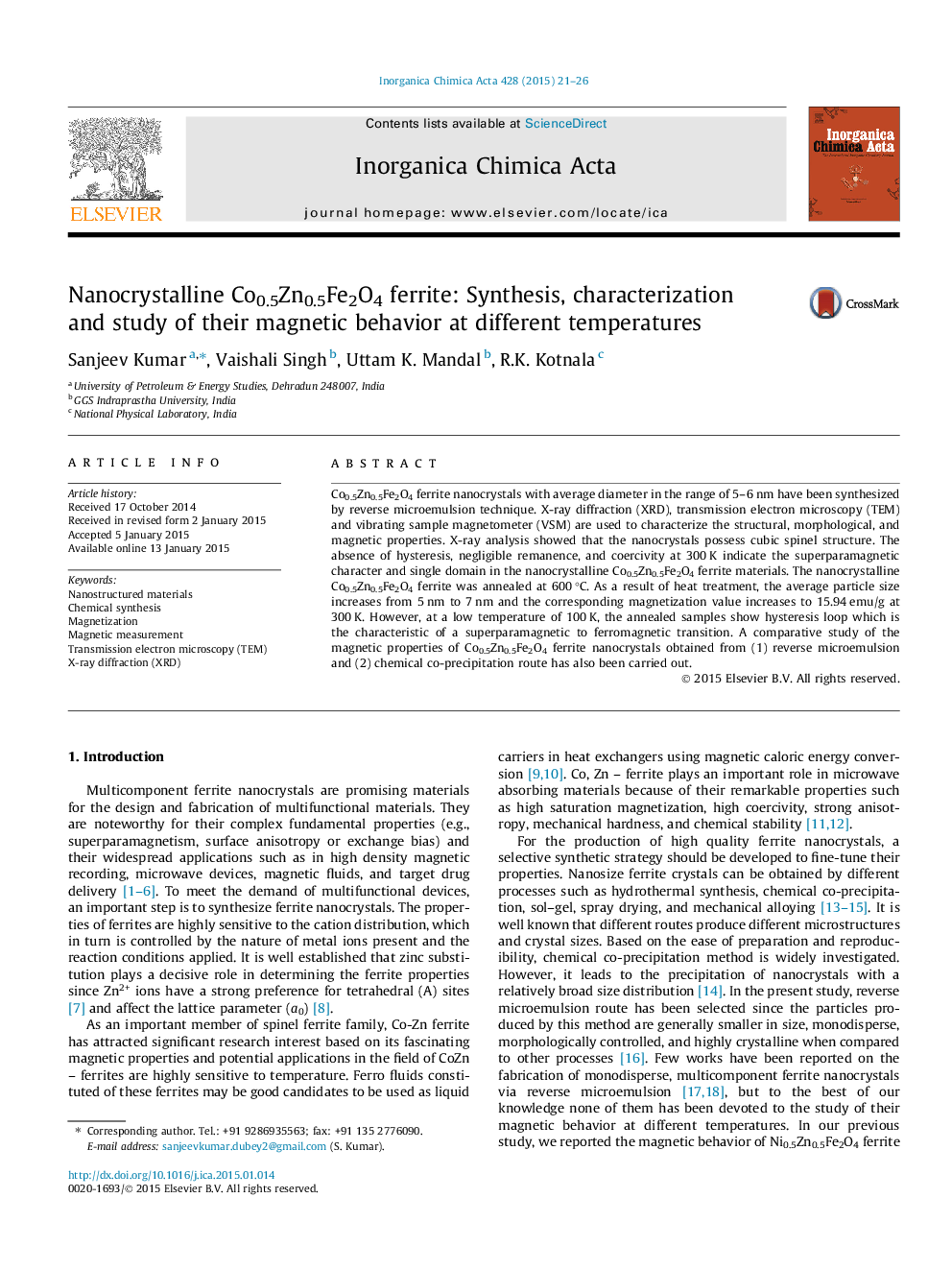
• Microemulsion and chemical precipitation have been used for the synthesis of said nanoparticle.
• Comparative studied carried out for the both method.
• Magnetism properties also studied at different temperature and established that microemulsion is the better process.
Co0.5Zn0.5Fe2O4 ferrite nanocrystals with average diameter in the range of 5–6 nm have been synthesized by reverse microemulsion technique. X-ray diffraction (XRD), transmission electron microscopy (TEM) and vibrating sample magnetometer (VSM) are used to characterize the structural, morphological, and magnetic properties. X-ray analysis showed that the nanocrystals possess cubic spinel structure. The absence of hysteresis, negligible remanence, and coercivity at 300 K indicate the superparamagnetic character and single domain in the nanocrystalline Co0.5Zn0.5Fe2O4 ferrite materials. The nanocrystalline Co0.5Zn0.5Fe2O4 ferrite was annealed at 600 °C. As a result of heat treatment, the average particle size increases from 5 nm to 7 nm and the corresponding magnetization value increases to 15.94 emu/g at 300 K. However, at a low temperature of 100 K, the annealed samples show hysteresis loop which is the characteristic of a superparamagnetic to ferromagnetic transition. A comparative study of the magnetic properties of Co0.5Zn0.5Fe2O4 ferrite nanocrystals obtained from (1) reverse microemulsion and (2) chemical co-precipitation route has also been carried out.
Magnetic properties and morphology (inset) of Co0.5Zn0.5Fe2O4 ferrite nanocrystals synthesized by (A) reverse microemulsion and (B) chemical co-precipitation at different temperatures.Figure optionsDownload as PowerPoint slide
Journal: Inorganica Chimica Acta - Volume 428, 24 March 2015, Pages 21–26