| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1311300 | 975310 | 2010 | 11 صفحه PDF | دانلود رایگان |
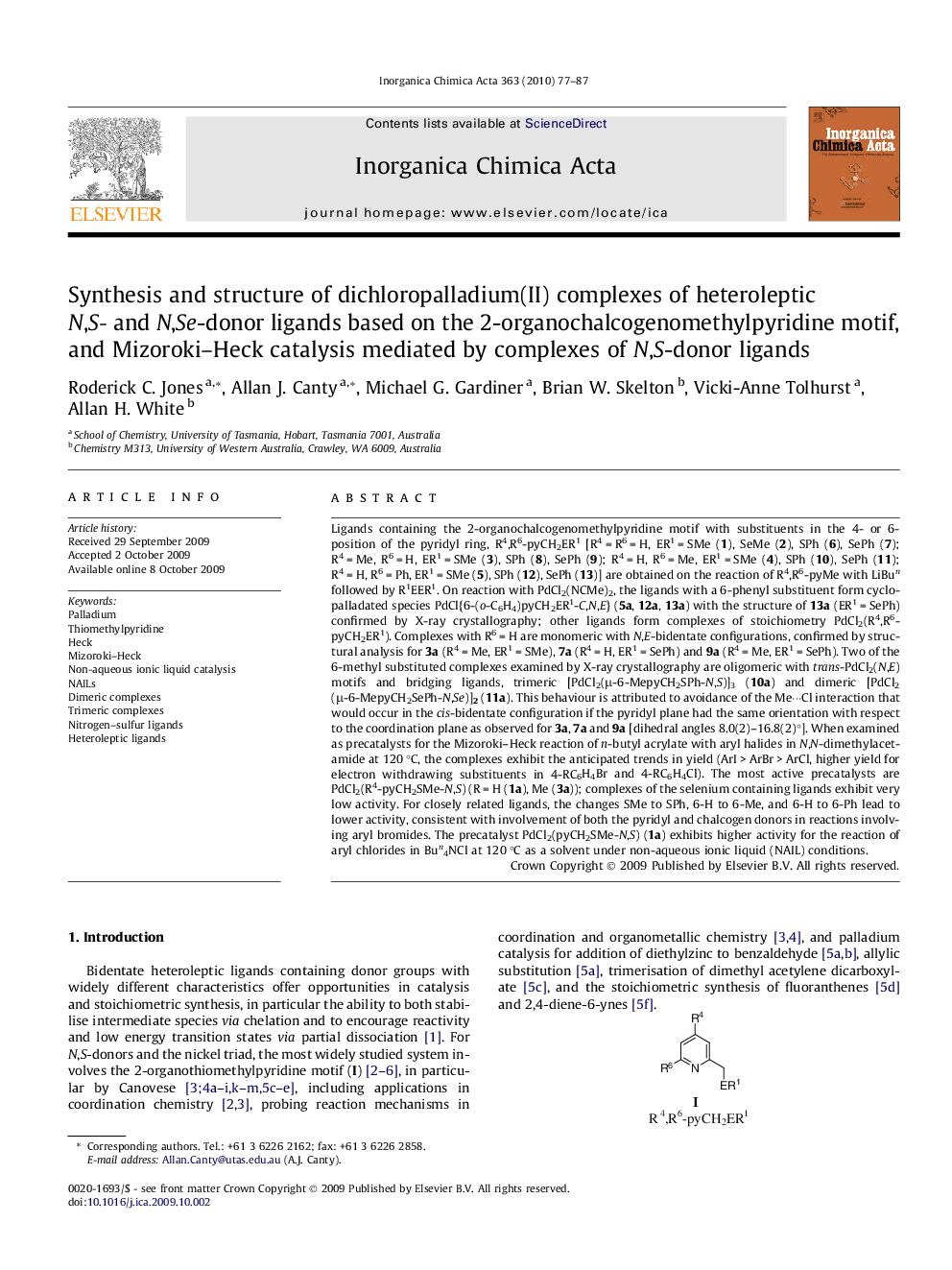
Ligands containing the 2-organochalcogenomethylpyridine motif with substituents in the 4- or 6-position of the pyridyl ring, R4,R6-pyCH2ER1 [R4 = R6 = H, ER1 = SMe (1), SeMe (2), SPh (6), SePh (7); R4 = Me, R6 = H, ER1 = SMe (3), SPh (8), SePh (9); R4 = H, R6 = Me, ER1 = SMe (4), SPh (10), SePh (11); R4 = H, R6 = Ph, ER1 = SMe (5), SPh (12), SePh (13)] are obtained on the reaction of R4,R6-pyMe with LiBun followed by R1EER1. On reaction with PdCl2(NCMe)2, the ligands with a 6-phenyl substituent form cyclopalladated species PdCl{6-(o-C6H4)pyCH2ER1-C,N,E} (5a, 12a, 13a) with the structure of 13a (ER1 = SePh) confirmed by X-ray crystallography; other ligands form complexes of stoichiometry PdCl2(R4,R6-pyCH2ER1). Complexes with R6 = H are monomeric with N,E-bidentate configurations, confirmed by structural analysis for 3a (R4 = Me, ER1 = SMe), 7a (R4 = H, ER1 = SePh) and 9a (R4 = Me, ER1 = SePh). Two of the 6-methyl substituted complexes examined by X-ray crystallography are oligomeric with trans-PdCl2(N,E) motifs and bridging ligands, trimeric [PdCl2(μ-6-MepyCH2SPh-N,S)]3 (10a) and dimeric [PdCl2(μ-6-MepyCH2SePh-N,Se)]2 (11a). This behaviour is attributed to avoidance of the Me···Cl interaction that would occur in the cis-bidentate configuration if the pyridyl plane had the same orientation with respect to the coordination plane as observed for 3a, 7a and 9a [dihedral angles 8.0(2)–16.8(2)°]. When examined as precatalysts for the Mizoroki–Heck reaction of n-butyl acrylate with aryl halides in N,N-dimethylacetamide at 120 °C, the complexes exhibit the anticipated trends in yield (ArI > ArBr > ArCl, higher yield for electron withdrawing substituents in 4-RC6H4Br and 4-RC6H4Cl). The most active precatalysts are PdCl2(R4-pyCH2SMe-N,S) (R = H (1a), Me (3a)); complexes of the selenium containing ligands exhibit very low activity. For closely related ligands, the changes SMe to SPh, 6-H to 6-Me, and 6-H to 6-Ph lead to lower activity, consistent with involvement of both the pyridyl and chalcogen donors in reactions involving aryl bromides. The precatalyst PdCl2(pyCH2SMe-N,S) (1a) exhibits higher activity for the reaction of aryl chlorides in Bun4NCl at 120 °C as a solvent under non-aqueous ionic liquid (NAIL) conditions.
A diverse reactivity of 2-organochalcogenomethylpyridines toward PdCl2(NCMe)2 is observed, and an examination of complexes as precatalysts for the Heck reaction indicate highest activity for the 2-methylthiomethylpyridine and 4-methyl-2-methylthiomethylpyridines.Figure optionsDownload as PowerPoint slide
Journal: Inorganica Chimica Acta - Volume 363, Issue 1, 4 January 2010, Pages 77–87