| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1331392 | 1500118 | 2013 | 9 صفحه PDF | دانلود رایگان |
The hydrothermal treatment of uranium trioxide and methylenediphosphonic acid with a variety of amines (2,2-dipyridyl, triethylenediamine, ethylenediamine, and 1,10-phenanthroline) at 200 °C results in the crystallization of a series of layered uranium diphosphonate compounds, [C10H9N2]{UO2(H2O)[CH2(PO3)(PO3H)]} (Ubip2), [C6H14N2]{(UO2)2[CH2(PO3)(PO3H)]2·2H2O} (UDAB), [C2H10N2]2{(UO2)2(H2O)2[CH2(PO3)2]2·0.5H2O} (Uethyl), and [C12H9N2]{UO2(H2O)[CH2(PO3)(PO3H)]} (Uphen). The crystal structures of the compounds are based on UO7 units linked by methylenediphosphonate molecules to form two-dimensional anionic sheets in Ubip2 and UDAB, and one-dimensional anionic chains in Uethyl and Uphen, which are charge balanced by protonated amine molecules. Interaction of the amine molecules with phosphonate oxygens and water molecules results in extensive hydrogen bonding in the interlayer. These amine molecules serve both as structure-directing agents and charge-balancing cations for the anionic uranium phosphonate sheets and chains in the formation of the different coordination geometries and topologies of each structure. Reported herein are the syntheses, structural and spectroscopic characterization of the synthesized compounds.
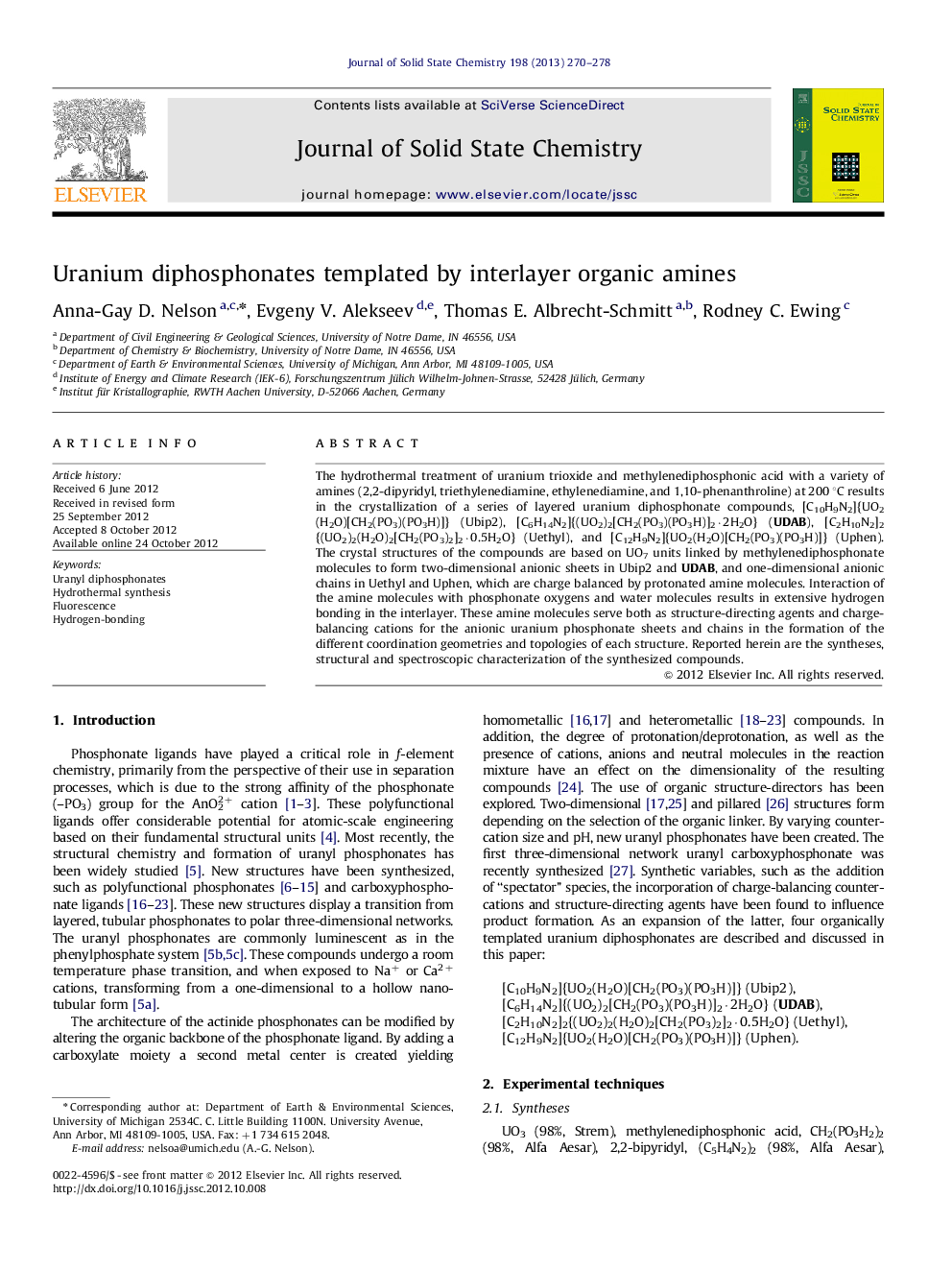
The Raman spectra of the synthesized compounds and an illustration of the stacking of the layers with the diprotonated triethylenediamine molecules in [C6H14N2]{(UO2)2[CH2(PO3)(PO3H)]2·2H2O} UDAB. Solvent water molecules are removed for clarity. The corresponding Raman spectra for the complexes synthesized is also shown. The structure is constructed from UO7 pentagonal bipyramids (yellow), oxygen=red, phosphorus=magenta, carbon=black, and nitrogen=blue. .Figure optionsDownload as PowerPoint slideHighlights
► Organic amines act both as charge-balancing and as structure-directing agents.
► Extensive hydrogen bonding interactions with solvent water molecules and amines.
► Altering the organic amine (size or flexibility) affects structure formation.
Journal: Journal of Solid State Chemistry - Volume 198, February 2013, Pages 270–278