| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1331409 | 1500118 | 2013 | 8 صفحه PDF | دانلود رایگان |
Within framework of the density functional theory, the structural and electronic properties of EuMg2 and its hydride EuMg2H6 have been investigated. The calculated structural parameters are in good agreement with the experimental results, and the obtained formation enthalpies indicate that the stability of EuMg2 is lower than that of EuMg2H6. The calculated electronic structures show that both compounds exhibit a strong spin polarization feature near the Fermi level owing to the localized Eu-f electrons, and the EuMg2H6 demonstrates mainly ionic characteristic. For thermodynamics of hydrogenation/dehydrogenation process of this system, the calculated reaction enthalpies along possible five reaction routes show that the most possible hydrogenation pathway is EuMg2+3H2→EuMg2H6, while the favorable dehydrogenation pathway is EuMg2H6→EuH2+2Mg+2H2.
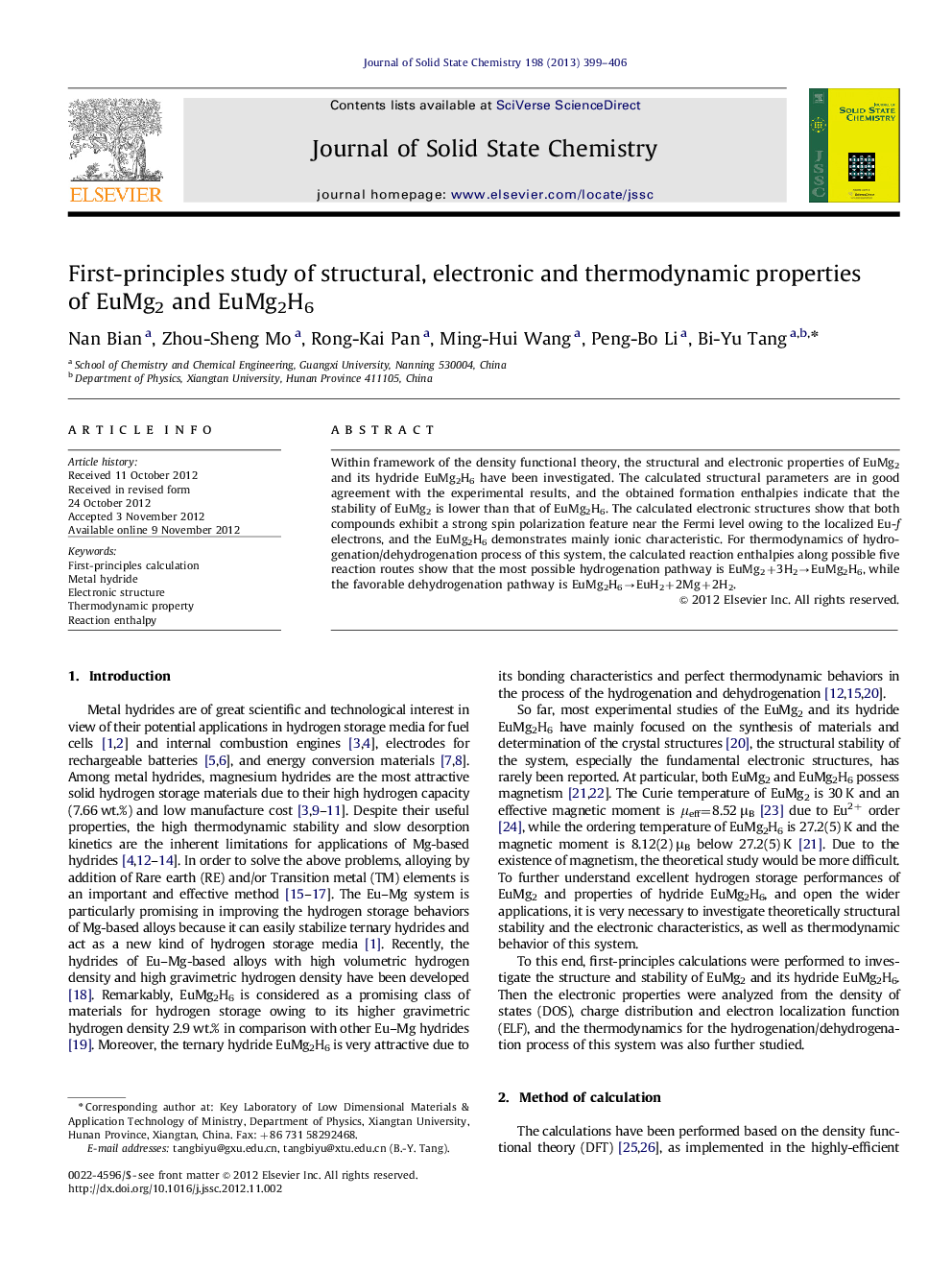
The charge difference distribution (e/Bohr3) (a) on (1010) plane of EuMg2, (b) on (110) and (c) (020) planes of its hydride EuMg2H6. Figure optionsDownload as PowerPoint slideHighlights
► The Eu-f electron has important effects on stability and hydrogen storage behavior.
► The hydride exhibits primary ionic characteristics.
► Three types of MgH bonding display also obvious ionic feature with some covalence.
► The dehydrogenation temperature of EuMg2H6 is further determined.
Journal: Journal of Solid State Chemistry - Volume 198, February 2013, Pages 399–406