| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1331421 | 1500118 | 2013 | 10 صفحه PDF | دانلود رایگان |
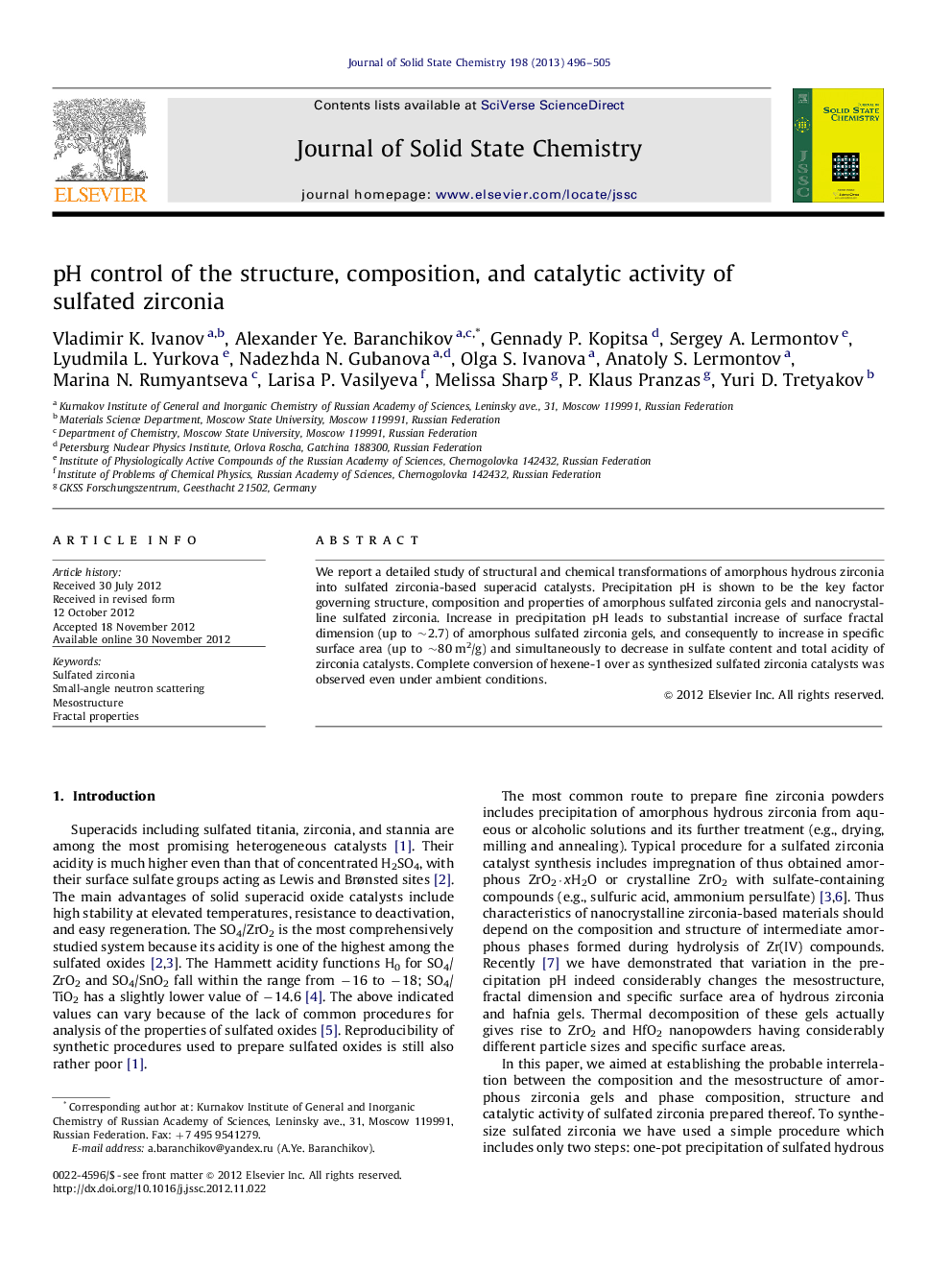
We report a detailed study of structural and chemical transformations of amorphous hydrous zirconia into sulfated zirconia-based superacid catalysts. Precipitation pH is shown to be the key factor governing structure, composition and properties of amorphous sulfated zirconia gels and nanocrystalline sulfated zirconia. Increase in precipitation pH leads to substantial increase of surface fractal dimension (up to ∼2.7) of amorphous sulfated zirconia gels, and consequently to increase in specific surface area (up to ∼80 m2/g) and simultaneously to decrease in sulfate content and total acidity of zirconia catalysts. Complete conversion of hexene-1 over as synthesized sulfated zirconia catalysts was observed even under ambient conditions.
Surface fractal dimension of amorphous sulfated zirconia and specific surface area and catalytic activity of crystalline sulfated zirconia as a function of precipitation pH. Figure optionsDownload as PowerPoint slideHighlights
► Structural transformation of amorphous hydrous zirconia into sulfated zirconia is studied.
► Precipitation pH controls surface fractal dimension of amorphous zirconia gels.
► Precipitation pH is the key factor governing properties of sulfated zirconia.
Journal: Journal of Solid State Chemistry - Volume 198, February 2013, Pages 496–505