| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1356676 | 981149 | 2008 | 5 صفحه PDF | دانلود رایگان |
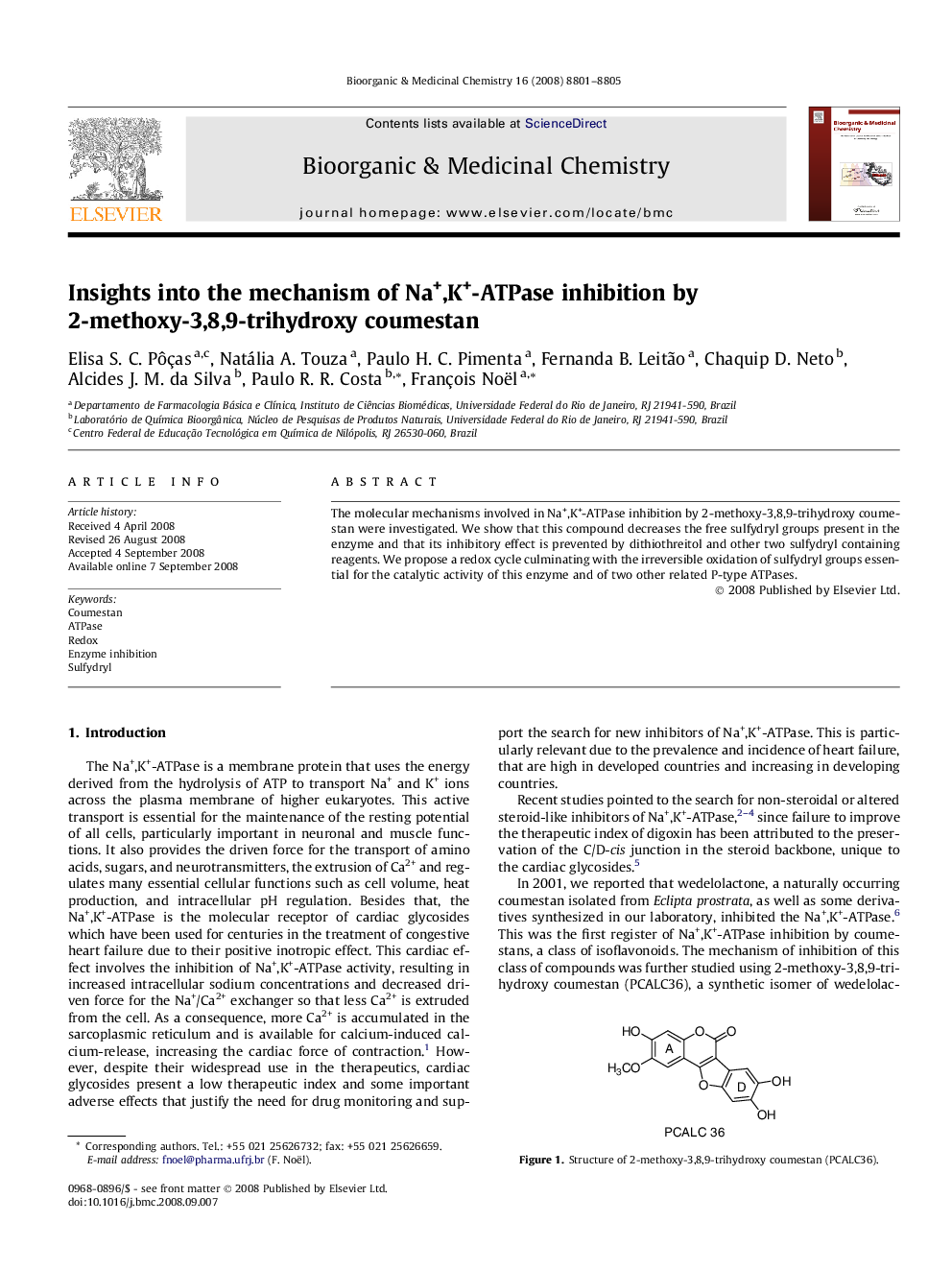
The molecular mechanisms involved in Na+,K+-ATPase inhibition by 2-methoxy-3,8,9-trihydroxy coumestan were investigated. We show that this compound decreases the free sulfydryl groups present in the enzyme and that its inhibitory effect is prevented by dithiothreitol and other two sulfydryl containing reagents. We propose a redox cycle culminating with the irreversible oxidation of sulfydryl groups essential for the catalytic activity of this enzyme and of two other related P-type ATPases.
The molecular mechanism involved in Na+,K+-ATPase inhibition by 2-methoxy-3,8,9-trihydroxy coumestan (PCALC36) was investigated. The results suggest that this compound irreversibly oxidizes important sulfydryl groups of the enzyme, explaining its poor selectivity toward other P-type ATPases.Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry - Volume 16, Issue 19, 1 October 2008, Pages 8801–8805