| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1359102 | 981384 | 2010 | 7 صفحه PDF | دانلود رایگان |
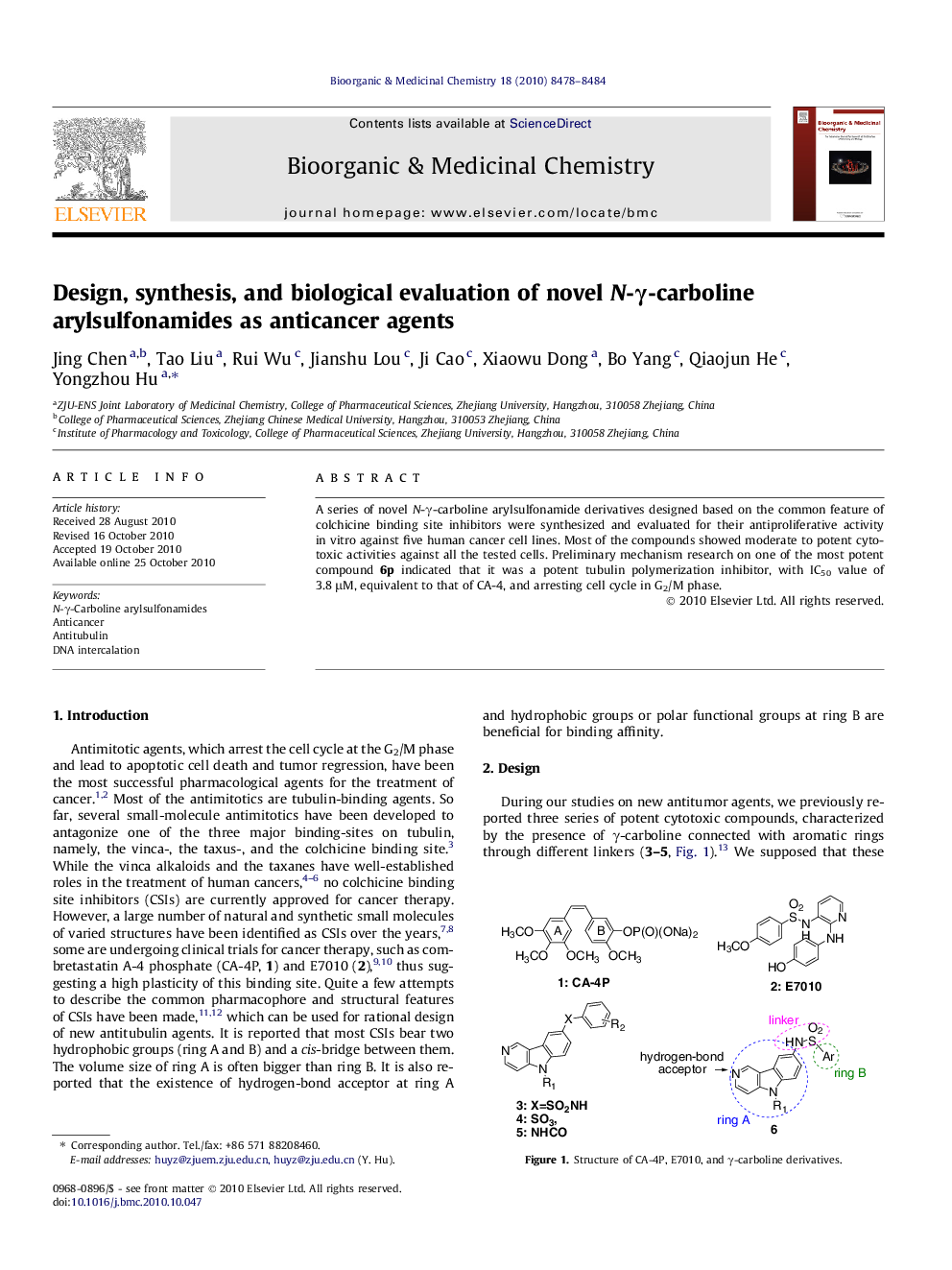
A series of novel N-γ-carboline arylsulfonamide derivatives designed based on the common feature of colchicine binding site inhibitors were synthesized and evaluated for their antiproliferative activity in vitro against five human cancer cell lines. Most of the compounds showed moderate to potent cytotoxic activities against all the tested cells. Preliminary mechanism research on one of the most potent compound 6p indicated that it was a potent tubulin polymerization inhibitor, with IC50 value of 3.8 μM, equivalent to that of CA-4, and arresting cell cycle in G2/M phase.
A series of novel N-γ-carboline arylsulfonamide derivatives designed, synthesized and evaluated for their cytotoxic activity, cell cycle effects, tubulin polymerization inhibition, and DNA intercalation.Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry - Volume 18, Issue 24, 15 December 2010, Pages 8478–8484