| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1360526 | 981438 | 2008 | 7 صفحه PDF | دانلود رایگان |
Diterpen labd-13(E)-ene-8a,15-diol (1) is a natural product found to possess potential cytotoxic and cytostatic effects against human cancer cell lines. Adenylyl cyclases (ACs) are promising pharmacological targets for treating heart failure, cancer, and psychosis. It has been demonstrated that forskolin is a potent adenylyl cyclase activator. Labdane 1 belongs to same family as forskolin. Its conformational properties are explored using a combination of 1D, 2D NMR spectroscopy, and molecular modeling techniques. The derived low energy conformers are subjected to docking calculations aiming to reveal similarities and differences in the binding mode between 1 and forskolin. Additionally, docking calculations performed on the 1α,9α-OH and 1α-OH derivatives of 1 suggest major contribution of 1α position in increasing binding affinity. This information may be of paramount importance to medicinal chemists who are interested in the synthesis of proposed analogs and test the docking results through in vitro experiments.
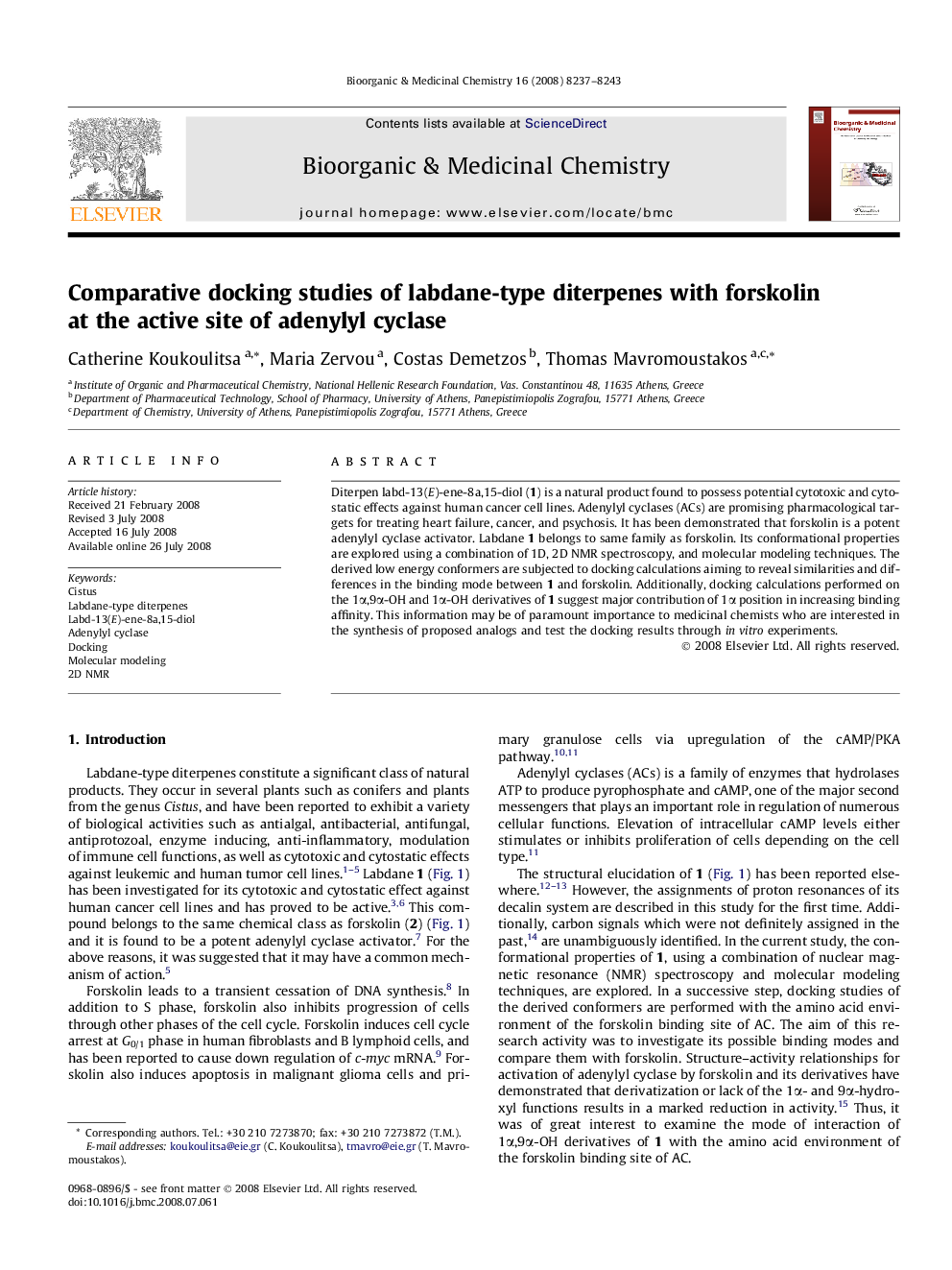
Best binding poses of the two enantiomers of labd-13(E)-ene-8a,15-diol (1a, colored yellow, and 1b, colored blue) in the forskolin binding site of AC.Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry - Volume 16, Issue 17, 1 September 2008, Pages 8237–8243