| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1361452 | 981463 | 2008 | 16 صفحه PDF | دانلود رایگان |
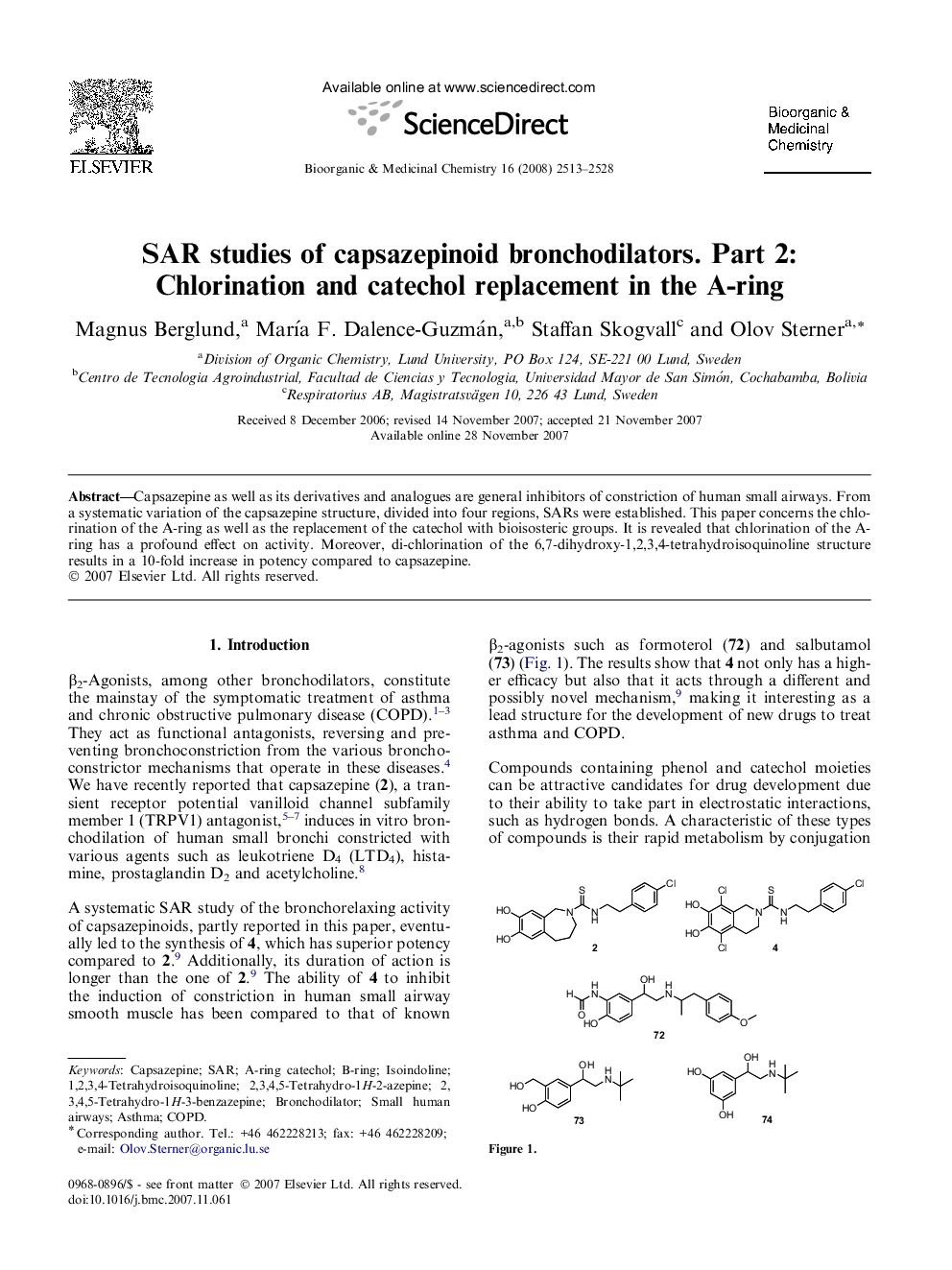
Capsazepine as well as its derivatives and analogues are general inhibitors of constriction of human small airways. From a systematic variation of the capsazepine structure, divided into four regions, SARs were established. This paper concerns the chlorination of the A-ring as well as the replacement of the catechol with bioisosteric groups. It is revealed that chlorination of the A-ring has a profound effect on activity. Moreover, di-chlorination of the 6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline structure results in a 10-fold increase in potency compared to capsazepine.
The dependence of the bronchodilating activity in human small airways of capsazepine derivatives on the chlorination of the A-ring and the replacement of the catechol moiety with bioisosteric groups has been investigated.Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry - Volume 16, Issue 5, 1 March 2008, Pages 2513–2528