| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1363950 | 981525 | 2009 | 5 صفحه PDF | دانلود رایگان |
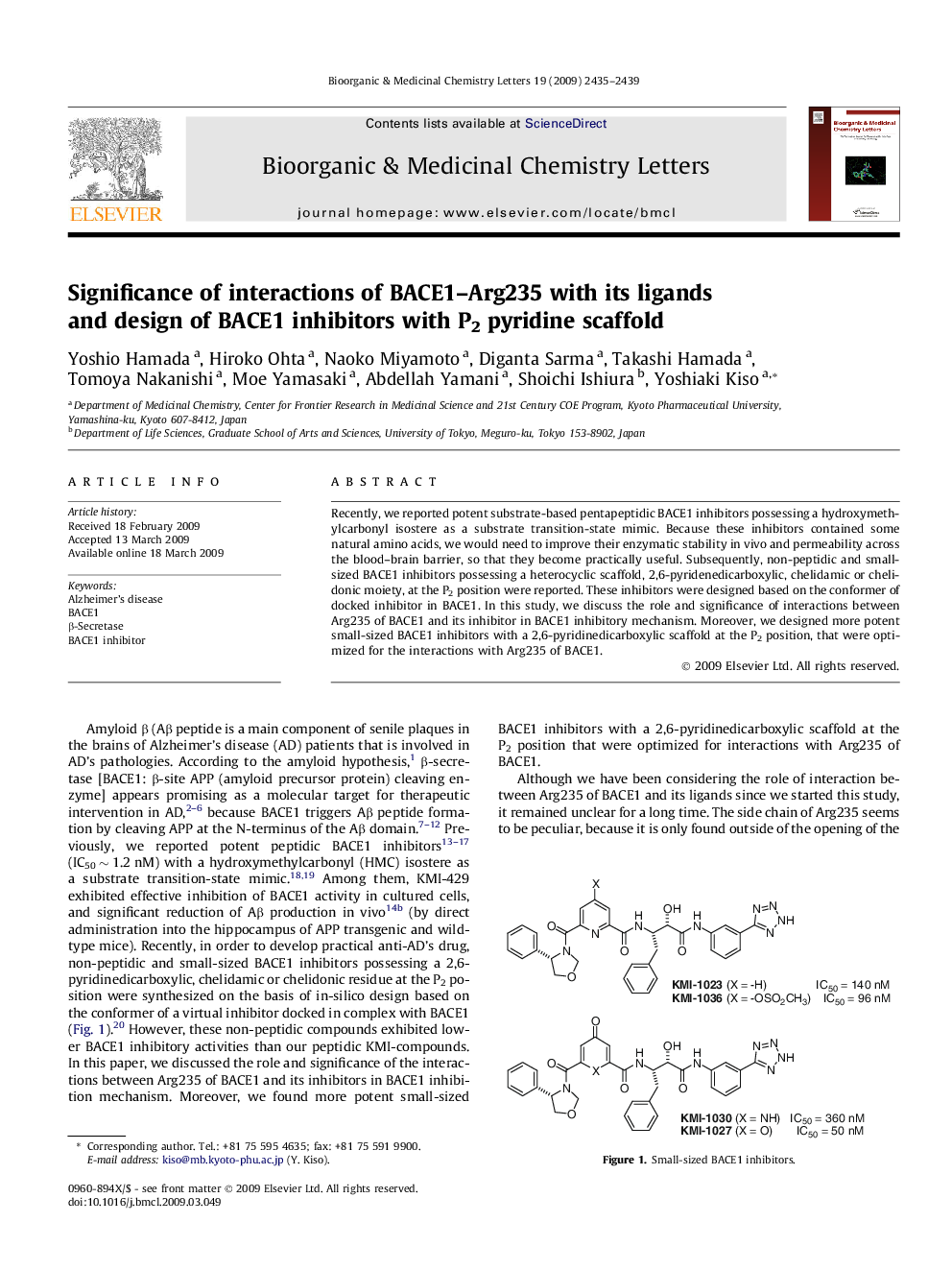
Recently, we reported potent substrate-based pentapeptidic BACE1 inhibitors possessing a hydroxymethylcarbonyl isostere as a substrate transition-state mimic. Because these inhibitors contained some natural amino acids, we would need to improve their enzymatic stability in vivo and permeability across the blood–brain barrier, so that they become practically useful. Subsequently, non-peptidic and small-sized BACE1 inhibitors possessing a heterocyclic scaffold, 2,6-pyridenedicarboxylic, chelidamic or chelidonic moiety, at the P2 position were reported. These inhibitors were designed based on the conformer of docked inhibitor in BACE1. In this study, we discuss the role and significance of interactions between Arg235 of BACE1 and its inhibitor in BACE1 inhibitory mechanism. Moreover, we designed more potent small-sized BACE1 inhibitors with a 2,6-pyridinedicarboxylic scaffold at the P2 position, that were optimized for the interactions with Arg235 of BACE1.
Following our hypothesis concerning the role of interaction of BACE1–Arg235 with its substrates/inhibitors, we designed a series of small-sized potent BACE1 inhibitors.Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry Letters - Volume 19, Issue 9, 1 May 2009, Pages 2435–2439