| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1371347 | 981843 | 2010 | 5 صفحه PDF | دانلود رایگان |
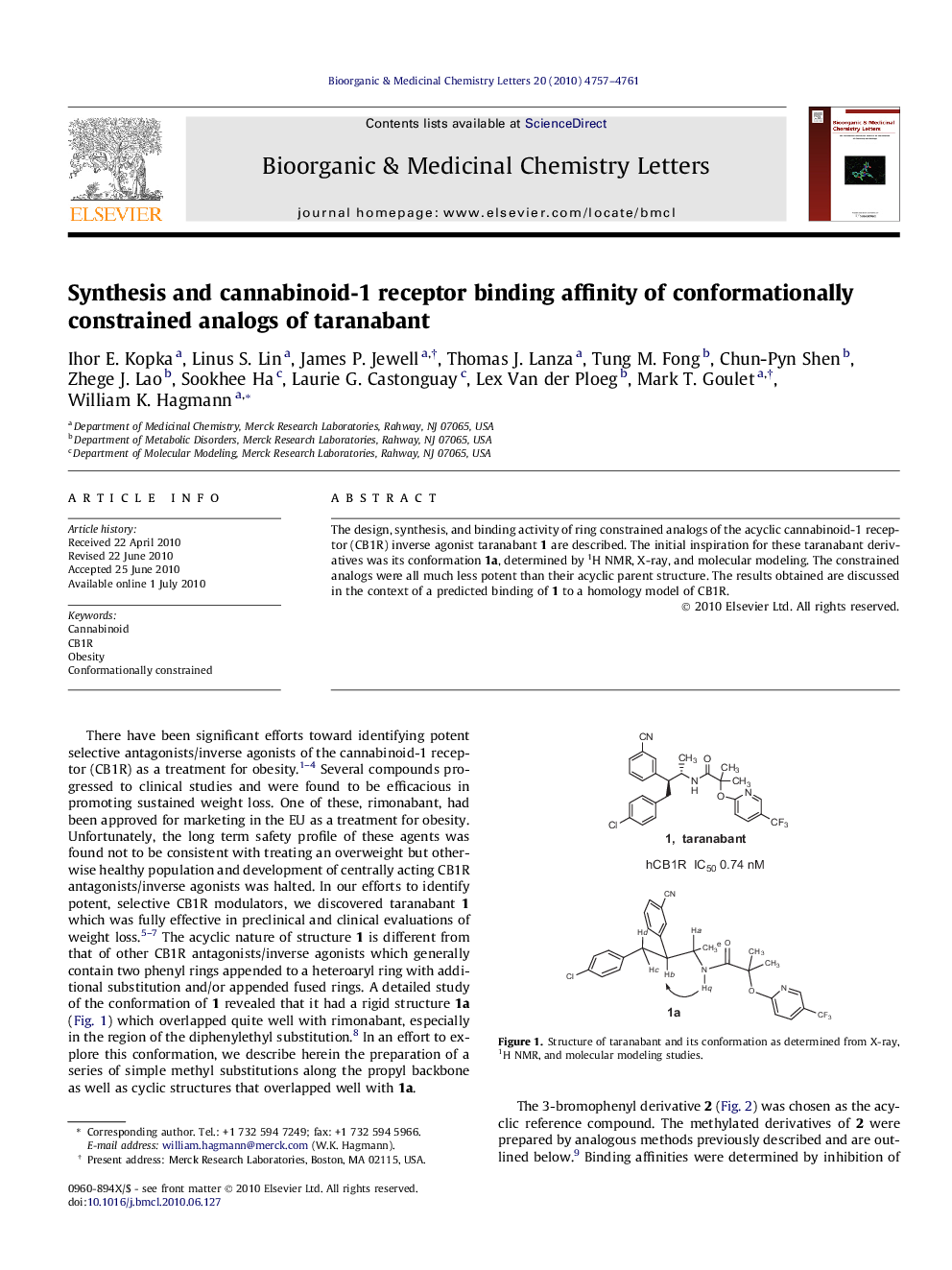
The design, synthesis, and binding activity of ring constrained analogs of the acyclic cannabinoid-1 receptor (CB1R) inverse agonist taranabant 1 are described. The initial inspiration for these taranabant derivatives was its conformation 1a, determined by 1H NMR, X-ray, and molecular modeling. The constrained analogs were all much less potent than their acyclic parent structure. The results obtained are discussed in the context of a predicted binding of 1 to a homology model of CB1R.
The design, synthesis, and binding activity of ring constrained analogs of the acyclic cannabinoid-1 receptor (CB1R) inverse agonist taranabant 1 are described. The initial inspiration for these taranabant derivatives was its conformation 1a, determined by 1H NMR, X-ray, and molecular modeling. The constrained analogs were all much less potent than their acyclic parent structure.Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry Letters - Volume 20, Issue 16, 15 August 2010, Pages 4757–4761