| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1373691 | 1500606 | 2016 | 12 صفحه PDF | دانلود رایگان |
• Synthesized and fabricated a novel transdermal film based device for the controlled release of lidocaine.
• The device possess excellent skin adhesiveness, mechanical stability, storage stability and less skin irritation potential.
• The fabricated film made from AHA/CS-g-GMA-g-BMA showed very good patient compliance and environmental stability.
• The transdermal film showed 68% drug release within 24 h.
A novel efficient transdermal (TD) lidocaine (LD) delivery device based on chitosan (CS) and hyaluronic acid (HA) was successfully developed in the present investigation. CS was grafted with glycidyl methacrylate (GMA) and butyl methacrylate (BMA) to fabricate a versatile material with improved adhesion and mechanical properties. HA was hydrophobically modified by covalently conjugating 3-(dimethylamino)-1-propylamine (DMPA) to encapsulate poorly water soluble LD and was uniformly dispersed in modified CS matrix. The prepared materials were characterized through FTIR, NMR, XRD, SEM, TEM and tensile assay. The dispersion of amine functionalized HA (AHA) on modified CS matrix offered strong matrix – filler interaction, which improved the mechanical properties and drug retention behavior of the device. In vitro skin permeation study of LD was performed with modified Franz diffusion cell using rat skin and exhibited controlled release. The influence of storage time on release profile was investigated and demonstrated that after the initial burst, LD release profile of the device after 30 and 60 days storage was identical to that of a device which was not stored. In vivo skin adhesion test and skin irritation assay in human subjects, water vapor permeability and environmental fitness test was performed to judge its application in biomedical field. All results displayed that the fabricated device is a potential candidate for TD LD administration to the systemic circulation.
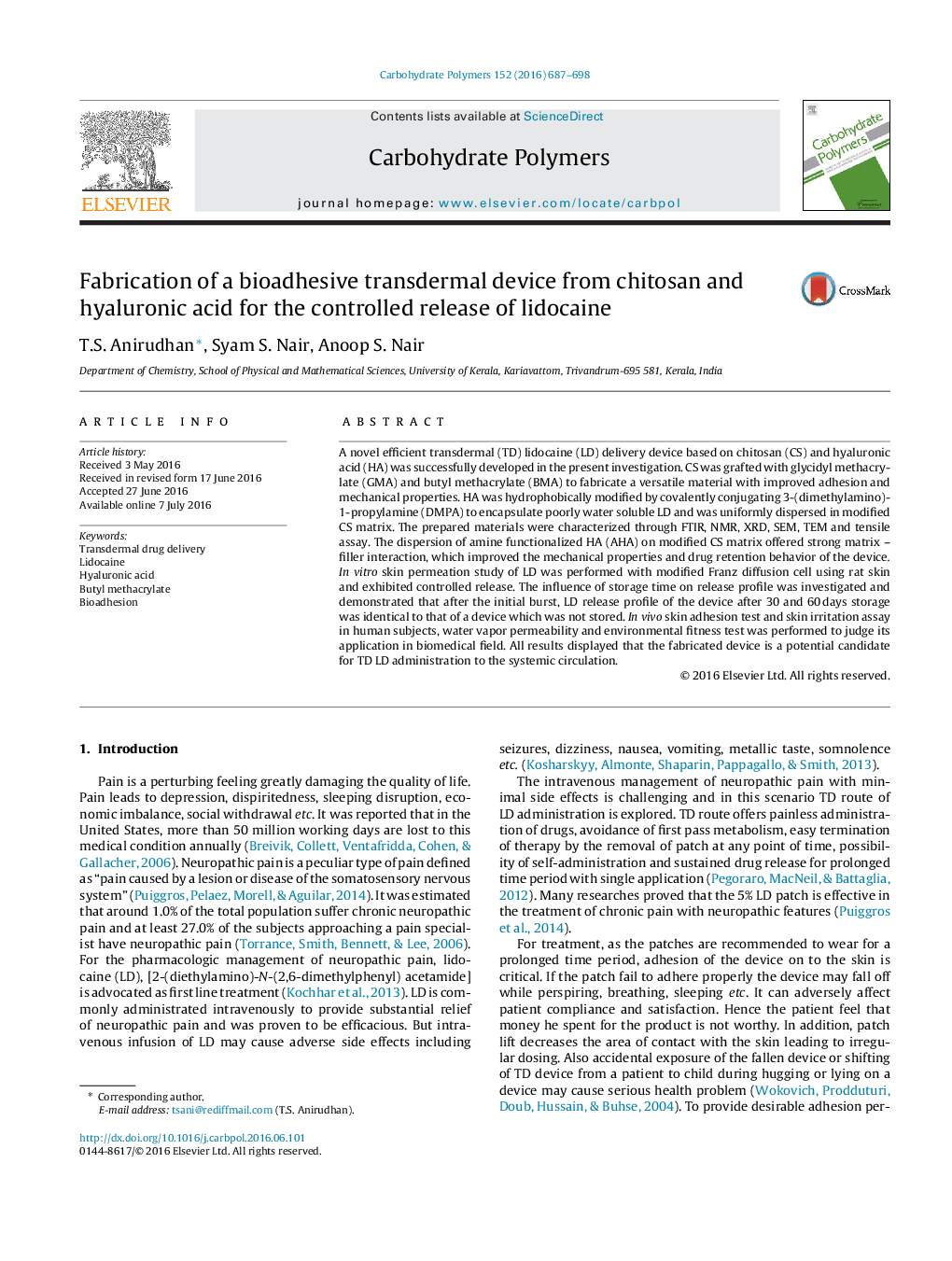
Schematic representation of A) fabrication of lidocaine loaded transdermal device, B) release of lidocaine from the device across stratum corneum.Figure optionsDownload as PowerPoint slide
Journal: Carbohydrate Polymers - Volume 152, 5 November 2016, Pages 687–698