| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1374714 | 981923 | 2006 | 5 صفحه PDF | دانلود رایگان |
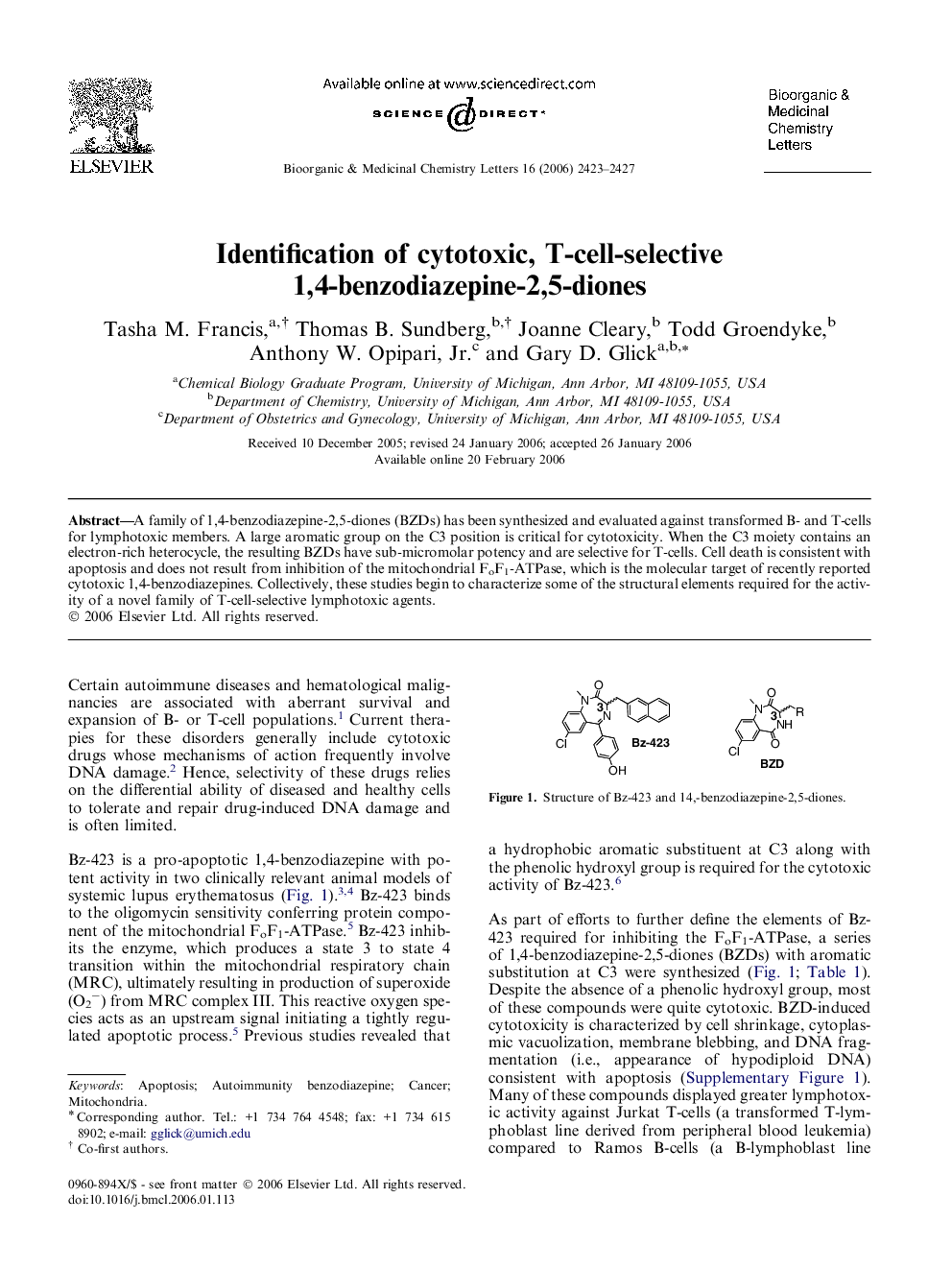
A family of 1,4-benzodiazepine-2,5-diones (BZDs) has been synthesized and evaluated against transformed B- and T-cells for lymphotoxic members. A large aromatic group on the C3 position is critical for cytotoxicity. When the C3 moiety contains an electron-rich heterocycle, the resulting BZDs have sub-micromolar potency and are selective for T-cells. Cell death is consistent with apoptosis and does not result from inhibition of the mitochondrial FoF1-ATPase, which is the molecular target of recently reported cytotoxic 1,4-benzodiazepines. Collectively, these studies begin to characterize some of the structural elements required for the activity of a novel family of T-cell-selective lymphotoxic agents.
A 1,4-benzodiazepine-2,5-dione (BZD) library was evaluated for lymphotoxic members. When the C3 substituent contains an electron-rich heterocycle, the resulting BZDs have sub-micromolar potency and are selective for T-cells.Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry Letters - Volume 16, Issue 9, 1 May 2006, Pages 2423–2427