| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1374793 | 981924 | 2008 | 4 صفحه PDF | دانلود رایگان |
A series of helicid analogues were synthesized and evaluated as tyrosinase inhibitors. The results demonstrated that some compounds had more potent inhibitory activities than arbutin (IC50 7.3 mM). In particular, compound 1c bearing 4,6-O-benzylidene substituent on the sugar moiety was found to be the most potent inhibitor with IC50 value of 0.052 mM. The inhibition kinetics analyzed by Lineweaver–Burk plots revealed that helicid analogues were competitive inhibitors. The Circular dichroism spectra indicated that those compounds induced conformational changes of mushroom tyrosinase upon binding.
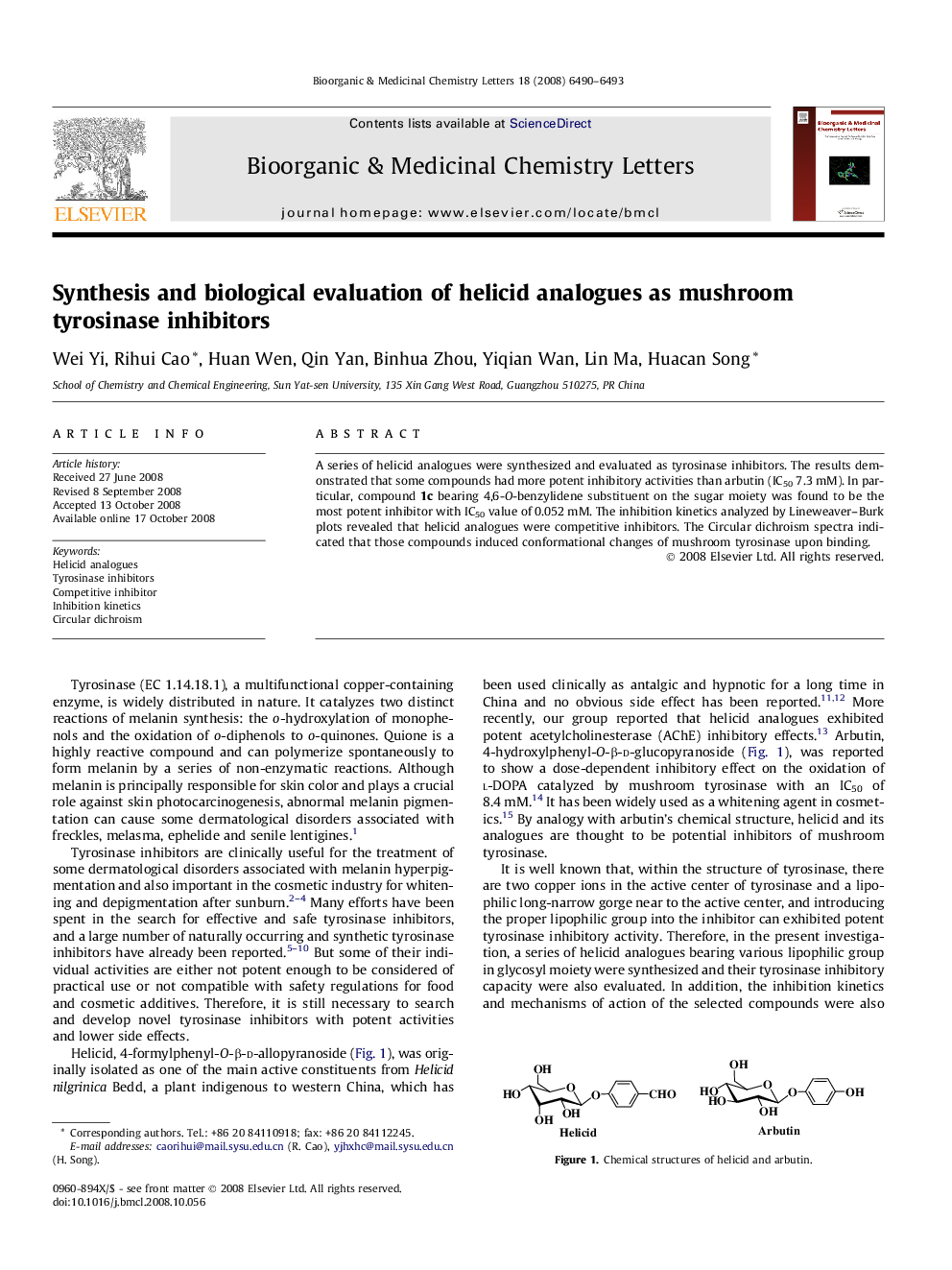
A series of helicid analogues were synthesized and their inhibitory activities on the diphenolase activity of mushroom tyrosinase were investigated. Compound 1c was found to be the most potent compound with IC50 value of 0.052 mΜ.Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry Letters - Volume 18, Issue 24, 15 December 2008, Pages 6490–6493