| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1401893 | 1501725 | 2015 | 7 صفحه PDF | دانلود رایگان |
• Three Ag complexes were obtained from 2-ethyl-3-pyrazine and aromatic dicarboxylates.
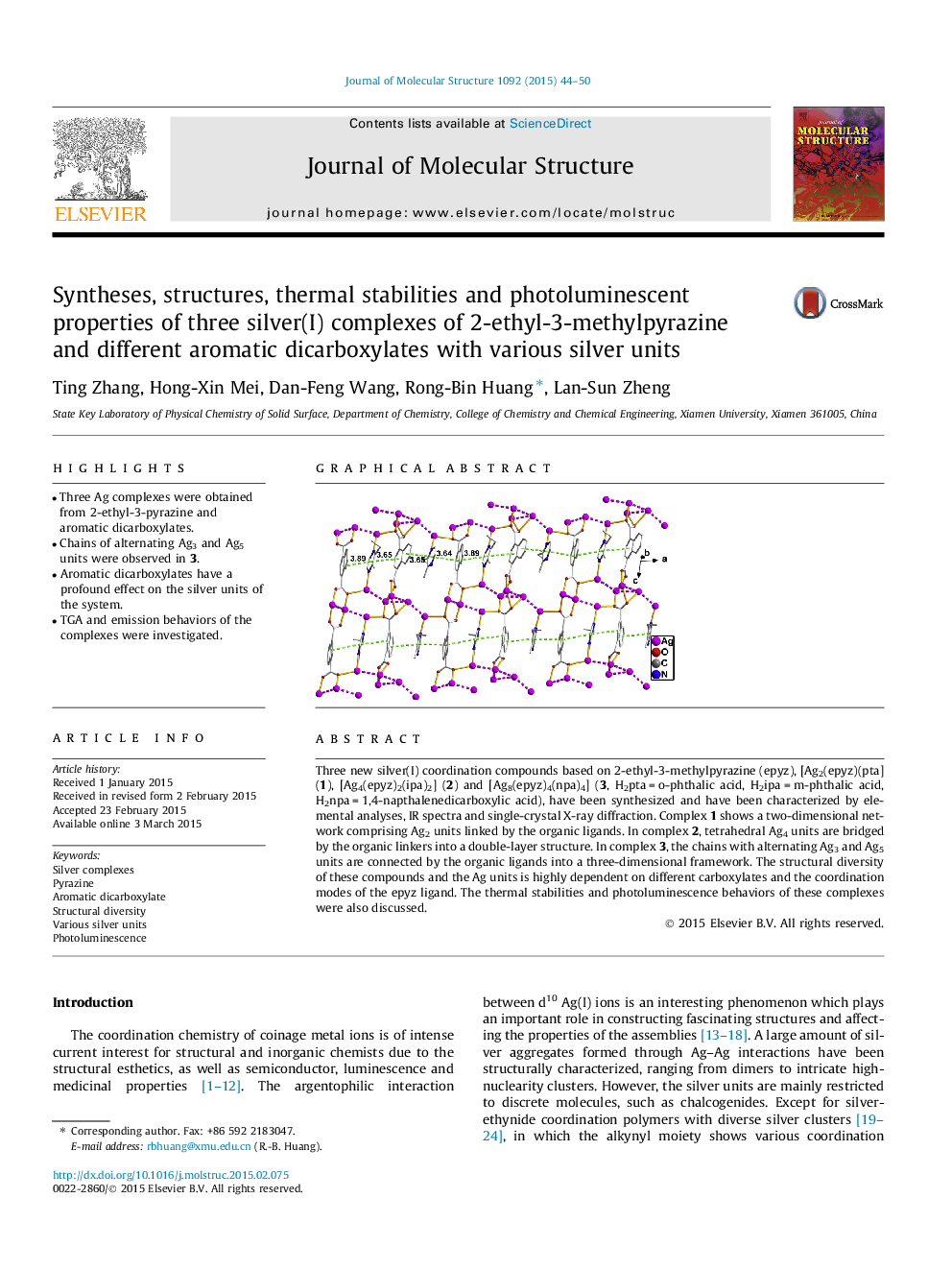
• Chains of alternating Ag3 and Ag5 units were observed in 3.
• Aromatic dicarboxylates have a profound effect on the silver units of the system.
• TGA and emission behaviors of the complexes were investigated.
Three new silver(I) coordination compounds based on 2-ethyl-3-methylpyrazine (epyz), [Ag2(epyz)(pta] (1), [Ag4(epyz)2(ipa)2] (2) and [Ag8(epyz)4(npa)4] (3, H2pta = o-phthalic acid, H2ipa = m-phthalic acid, H2npa = 1,4-napthalenedicarboxylic acid), have been synthesized and have been characterized by elemental analyses, IR spectra and single-crystal X-ray diffraction. Complex 1 shows a two-dimensional network comprising Ag2 units linked by the organic ligands. In complex 2, tetrahedral Ag4 units are bridged by the organic linkers into a double-layer structure. In complex 3, the chains with alternating Ag3 and Ag5 units are connected by the organic ligands into a three-dimensional framework. The structural diversity of these compounds and the Ag units is highly dependent on different carboxylates and the coordination modes of the epyz ligand. The thermal stabilities and photoluminescence behaviors of these complexes were also discussed.
Figure optionsDownload as PowerPoint slide
Journal: Journal of Molecular Structure - Volume 1092, 15 July 2015, Pages 44–50