| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1402140 | 1501738 | 2015 | 8 صفحه PDF | دانلود رایگان |
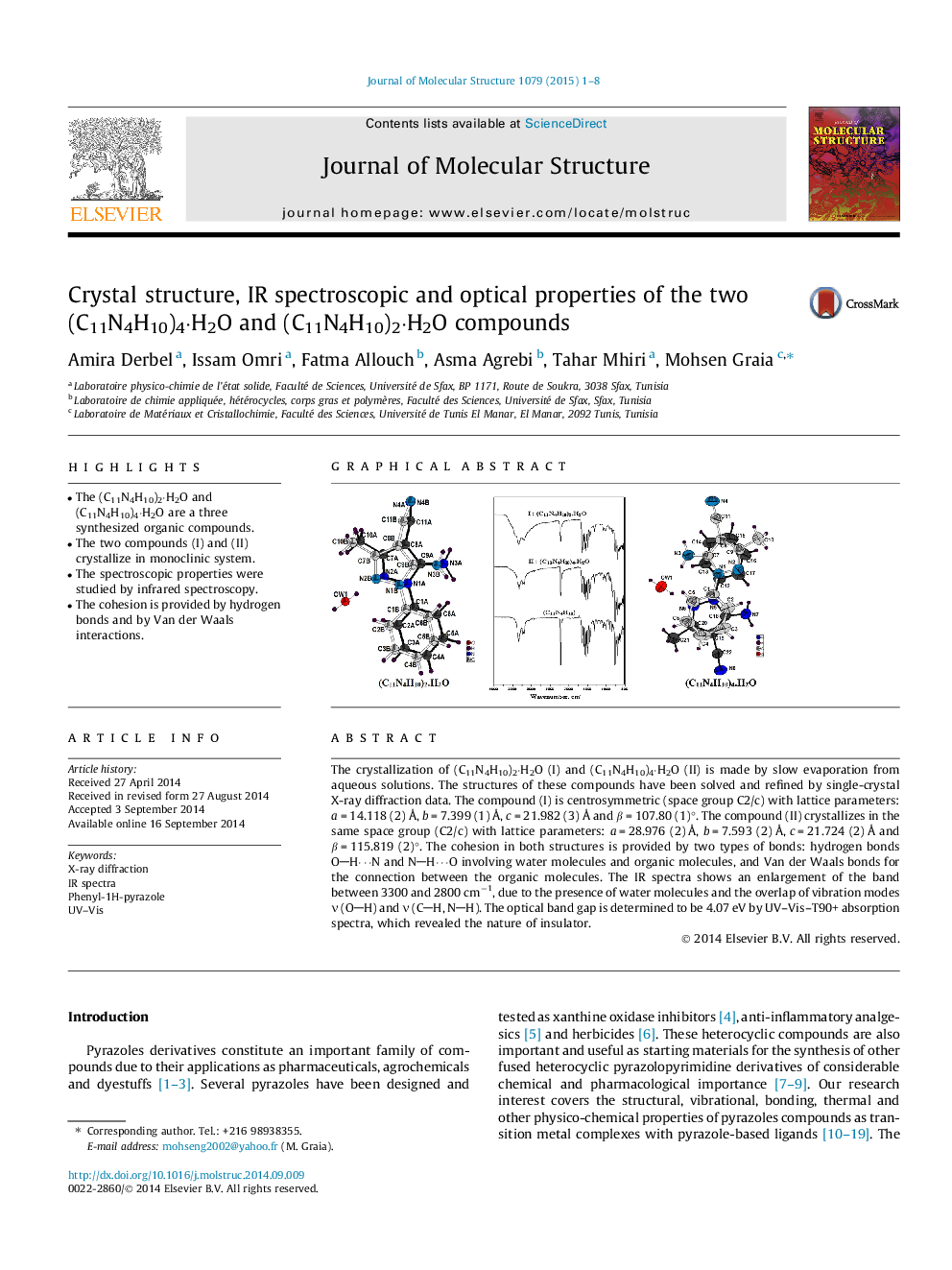
• The (C11N4H10)2·H2O and (C11N4H10)4·H2O are a three synthesized organic compounds.
• The two compounds (I) and (II) crystallize in monoclinic system.
• The spectroscopic properties were studied by infrared spectroscopy.
• The cohesion is provided by hydrogen bonds and by Van der Waals interactions.
The crystallization of (C11N4H10)2·H2O (I) and (C11N4H10)4·H2O (II) is made by slow evaporation from aqueous solutions. The structures of these compounds have been solved and refined by single-crystal X-ray diffraction data. The compound (I) is centrosymmetric (space group C2/c) with lattice parameters: a = 14.118 (2) Å, b = 7.399 (1) Å, c = 21.982 (3) Å and β = 107.80 (1)°. The compound (II) crystallizes in the same space group (C2/c) with lattice parameters: a = 28.976 (2) Å, b = 7.593 (2) Å, c = 21.724 (2) Å and β = 115.819 (2)°. The cohesion in both structures is provided by two types of bonds: hydrogen bonds OH⋯N and NH⋯O involving water molecules and organic molecules, and Van der Waals bonds for the connection between the organic molecules. The IR spectra shows an enlargement of the band between 3300 and 2800 cm−1, due to the presence of water molecules and the overlap of vibration modes ν (OH) and ν (CH, NH). The optical band gap is determined to be 4.07 eV by UV–Vis–T90+ absorption spectra, which revealed the nature of insulator.
Figure optionsDownload as PowerPoint slide
Journal: Journal of Molecular Structure - Volume 1079, 5 January 2015, Pages 1–8