| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1402152 | 1501738 | 2015 | 9 صفحه PDF | دانلود رایگان |
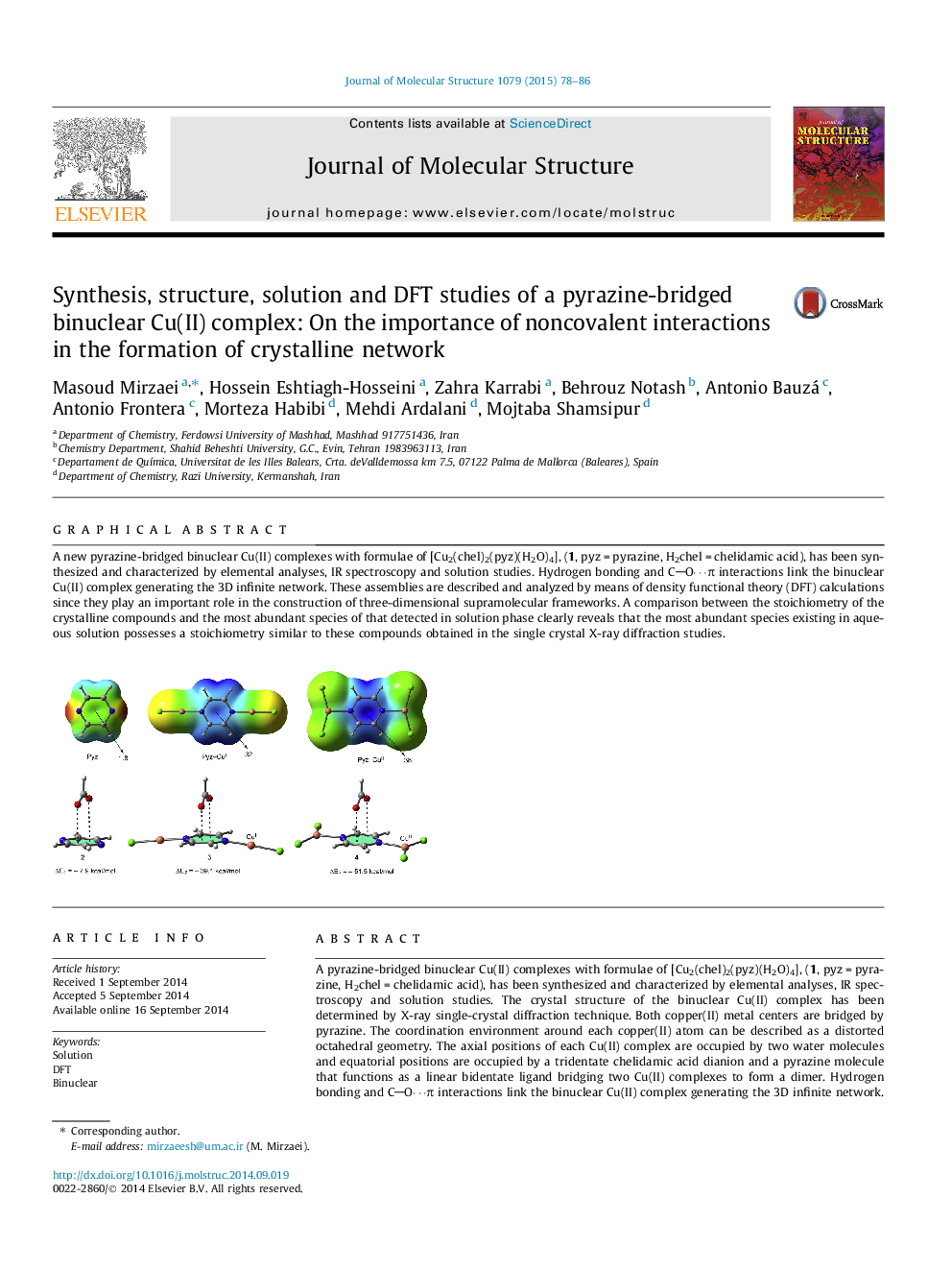
A pyrazine-bridged binuclear Cu(II) complexes with formulae of [Cu2(chel)2(pyz)(H2O)4], (1, pyz = pyrazine, H2chel = chelidamic acid), has been synthesized and characterized by elemental analyses, IR spectroscopy and solution studies. The crystal structure of the binuclear Cu(II) complex has been determined by X-ray single-crystal diffraction technique. Both copper(II) metal centers are bridged by pyrazine. The coordination environment around each copper(II) atom can be described as a distorted octahedral geometry. The axial positions of each Cu(II) complex are occupied by two water molecules and equatorial positions are occupied by a tridentate chelidamic acid dianion and a pyrazine molecule that functions as a linear bidentate ligand bridging two Cu(II) complexes to form a dimer. Hydrogen bonding and CO⋯π interactions link the binuclear Cu(II) complex generating the 3D infinite network. These assemblies are described and analyzed by means of density functional theory (DFT) calculations since they play an important role in the construction of three-dimensional supramolecular frameworks. The protonation constants of pyrazine and chelidamic acid as the building blocks of the proton transfer systems (H2chel-pyz) and their corresponding stability constants were determined by potentiometric studies. The stoichiometry and stability constants of H2chel-pyz complex with Cu2+ was investigated by potentiometric technique in aqueous solution. The results from solution studies were compared with the solid state data, in details.
A new pyrazine-bridged binuclear Cu(II) complexes with formulae of [Cu2(chel)2(pyz)(H2O)4], (1, pyz = pyrazine, H2chel = chelidamic acid), has been synthesized and characterized by elemental analyses, IR spectroscopy and solution studies. Hydrogen bonding and CO⋯π interactions link the binuclear Cu(II) complex generating the 3D infinite network. These assemblies are described and analyzed by means of density functional theory (DFT) calculations since they play an important role in the construction of three-dimensional supramolecular frameworks. A comparison between the stoichiometry of the crystalline compounds and the most abundant species of that detected in solution phase clearly reveals that the most abundant species existing in aqueous solution possesses a stoichiometry similar to these compounds obtained in the single crystal X-ray diffraction studies.Figure optionsDownload as PowerPoint slide
Journal: Journal of Molecular Structure - Volume 1079, 5 January 2015, Pages 78–86