| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1402182 | 1501738 | 2015 | 6 صفحه PDF | دانلود رایگان |
• The calculated absorption peaks agree well with the measured data.
• The lowest energy transitions are H–L transition with ππ* character.
• Solvent effect has effect on wavelength and oscillator strengths.
• The pyrrolo-C analogues retain the mode and strength of H-bonds.
• The results of CAM-B3LYP functional agree well with M062X functional.
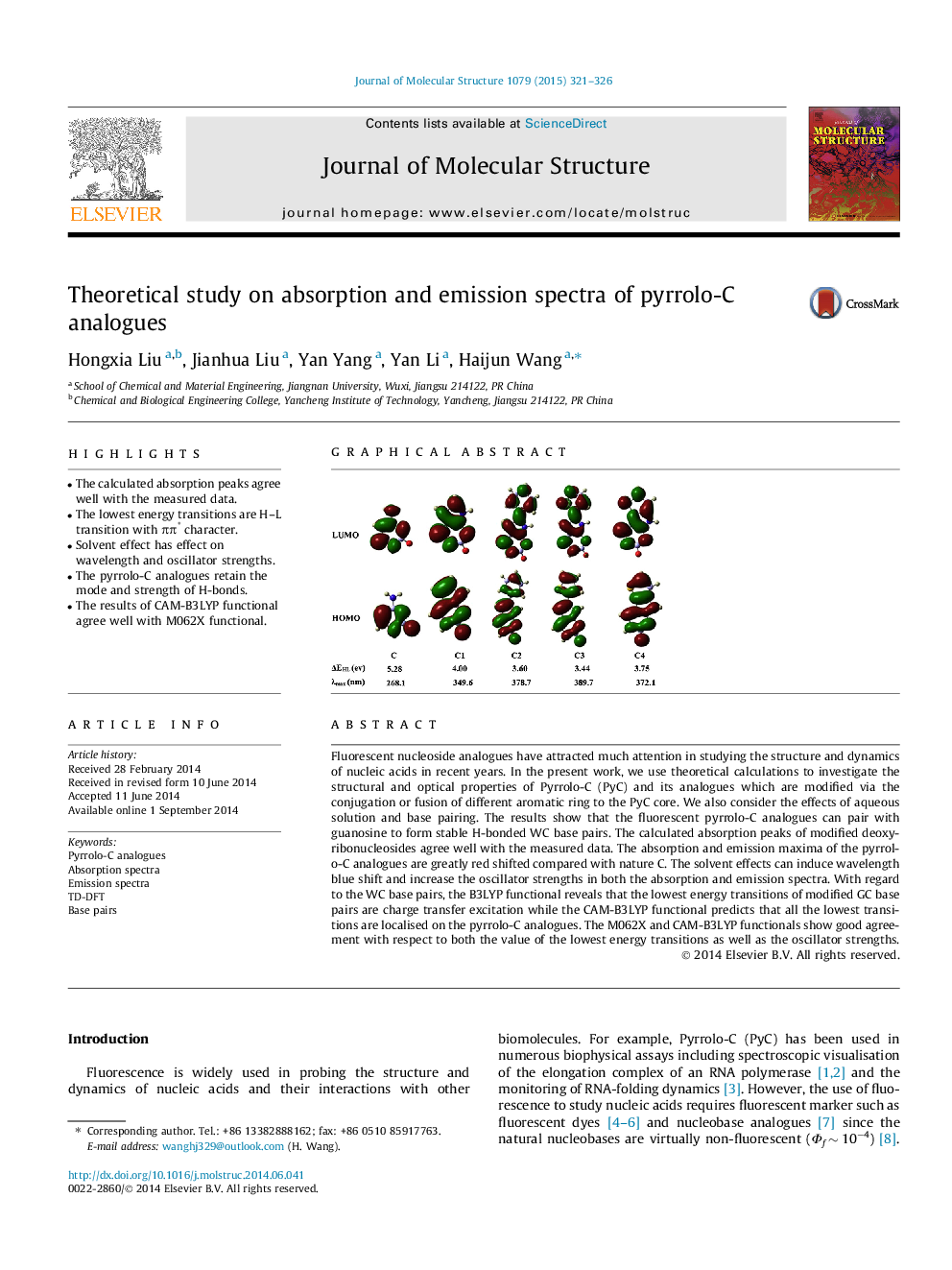
Fluorescent nucleoside analogues have attracted much attention in studying the structure and dynamics of nucleic acids in recent years. In the present work, we use theoretical calculations to investigate the structural and optical properties of Pyrrolo-C (PyC) and its analogues which are modified via the conjugation or fusion of different aromatic ring to the PyC core. We also consider the effects of aqueous solution and base pairing. The results show that the fluorescent pyrrolo-C analogues can pair with guanosine to form stable H-bonded WC base pairs. The calculated absorption peaks of modified deoxyribonucleosides agree well with the measured data. The absorption and emission maxima of the pyrrolo-C analogues are greatly red shifted compared with nature C. The solvent effects can induce wavelength blue shift and increase the oscillator strengths in both the absorption and emission spectra. With regard to the WC base pairs, the B3LYP functional reveals that the lowest energy transitions of modified GC base pairs are charge transfer excitation while the CAM-B3LYP functional predicts that all the lowest transitions are localised on the pyrrolo-C analogues. The M062X and CAM-B3LYP functionals show good agreement with respect to both the value of the lowest energy transitions as well as the oscillator strengths.
Figure optionsDownload as PowerPoint slide
Journal: Journal of Molecular Structure - Volume 1079, 5 January 2015, Pages 321–326