| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1402396 | 1501743 | 2014 | 8 صفحه PDF | دانلود رایگان |
• Novel stability constants were determined for binaries and ternary systems.
• Coordination mode was given and also new insights into the brain biochemistry.
• Ligand interactions in the ternary system may not be free for formation of ATP.
• Potentiometry and other techniques were used and minimum energy calculated.
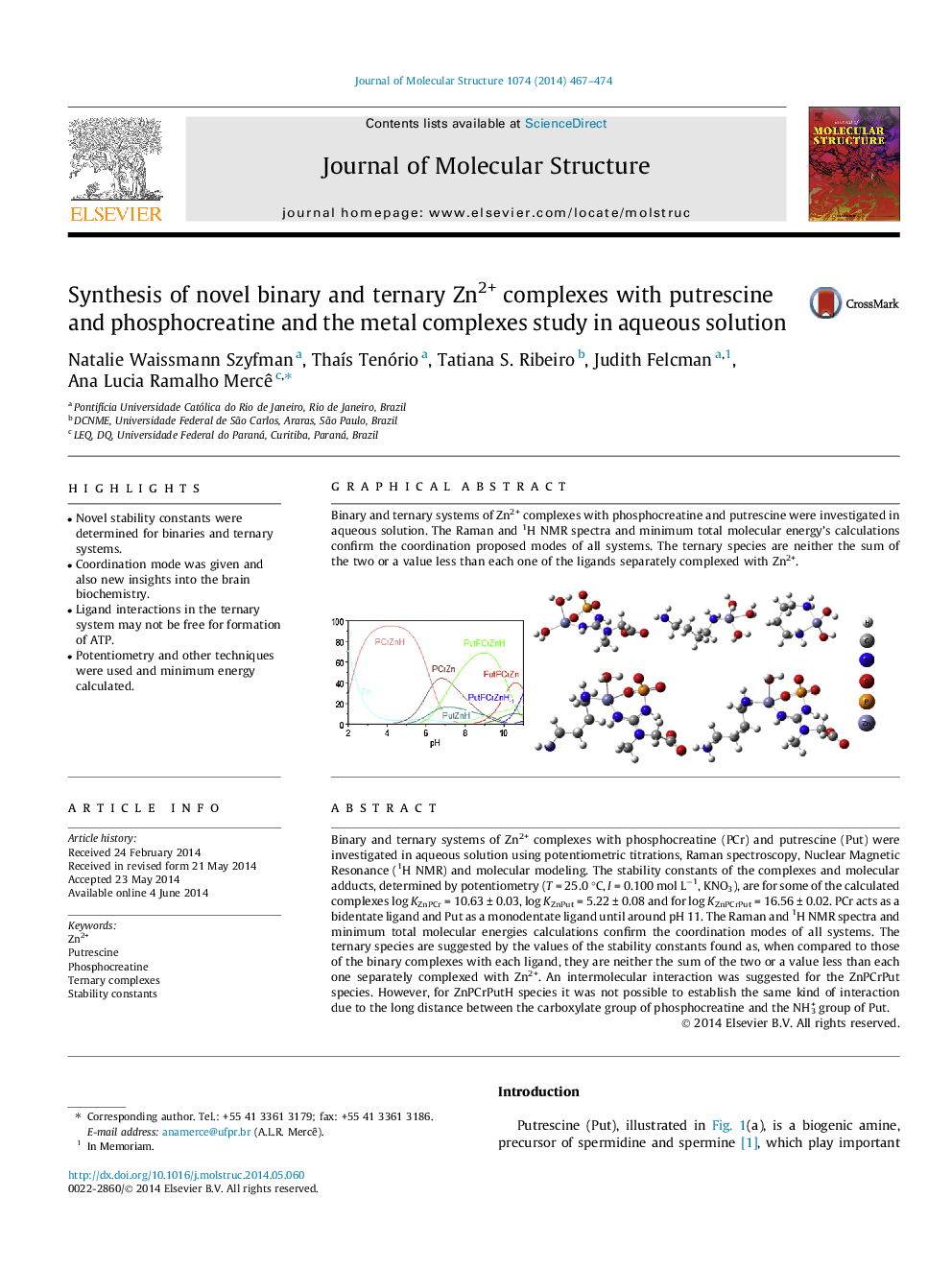
Binary and ternary systems of Zn2+ complexes with phosphocreatine (PCr) and putrescine (Put) were investigated in aqueous solution using potentiometric titrations, Raman spectroscopy, Nuclear Magnetic Resonance (1H NMR) and molecular modeling. The stability constants of the complexes and molecular adducts, determined by potentiometry (T = 25.0 °C, I = 0.100 mol L−1, KNO3), are for some of the calculated complexes log KZnPCr = 10.63 ± 0.03, log KZnPut = 5.22 ± 0.08 and for log KZnPCrPut = 16.56 ± 0.02. PCr acts as a bidentate ligand and Put as a monodentate ligand until around pH 11. The Raman and 1H NMR spectra and minimum total molecular energies calculations confirm the coordination modes of all systems. The ternary species are suggested by the values of the stability constants found as, when compared to those of the binary complexes with each ligand, they are neither the sum of the two or a value less than each one separately complexed with Zn2+. An intermolecular interaction was suggested for the ZnPCrPut species. However, for ZnPCrPutH species it was not possible to establish the same kind of interaction due to the long distance between the carboxylate group of phosphocreatine and the NH3+ group of Put.
Binary and ternary systems of Zn2+ complexes with phosphocreatine and putrescine were investigated in aqueous solution. The Raman and 1H NMR spectra and minimum total molecular energy’s calculations confirm the coordination proposed modes of all systems. The ternary species are neither the sum of the two or a value less than each one of the ligands separately complexed with Zn2+.Figure optionsDownload as PowerPoint slide
Journal: Journal of Molecular Structure - Volume 1074, 25 September 2014, Pages 467–474