| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1405096 | 1501747 | 2014 | 7 صفحه PDF | دانلود رایگان |
• Three d10 metal coordination polymers have been characterized.
• Effect of dicarboxylates on the structure of complexes was discussed.
• TG analysis confirms the stability of the coordination polymers.
• Luminescence properties of complexes have been investigated.
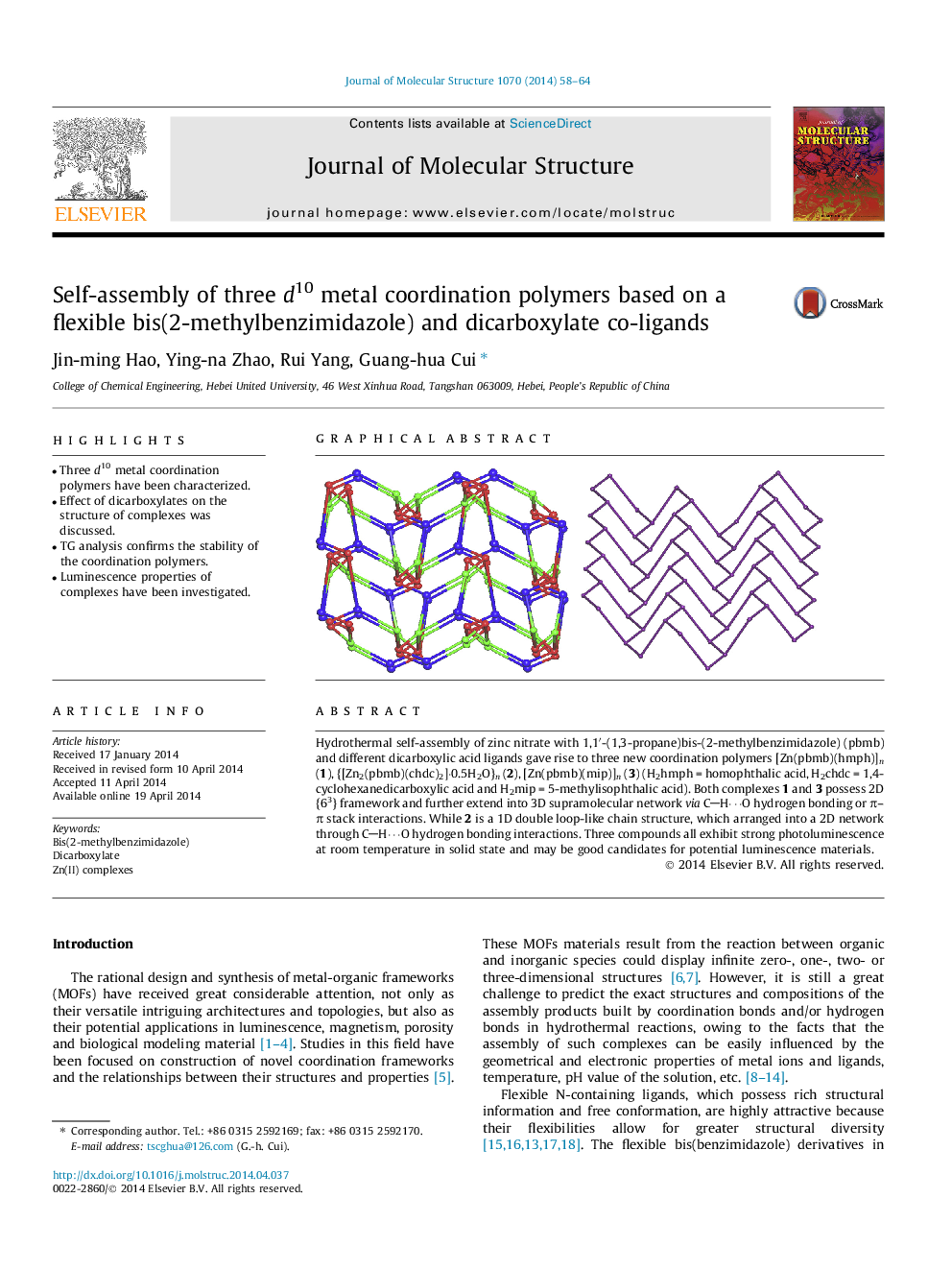
Hydrothermal self-assembly of zinc nitrate with 1,1′-(1,3-propane)bis-(2-methylbenzimidazole) (pbmb) and different dicarboxylic acid ligands gave rise to three new coordination polymers [Zn(pbmb)(hmph)]n (1), {[Zn2(pbmb)(chdc)2]⋅0.5H2O}n (2), [Zn(pbmb)(mip)]n (3) (H2hmph = homophthalic acid, H2chdc = 1,4-cyclohexanedicarboxylic acid and H2mip = 5-methylisophthalic acid). Both complexes 1 and 3 possess 2D {63} framework and further extend into 3D supramolecular network via CH⋯O hydrogen bonding or π–π stack interactions. While 2 is a 1D double loop-like chain structure, which arranged into a 2D network through CH⋯O hydrogen bonding interactions. Three compounds all exhibit strong photoluminescence at room temperature in solid state and may be good candidates for potential luminescence materials.
Figure optionsDownload as PowerPoint slide
Journal: Journal of Molecular Structure - Volume 1070, 24 July 2014, Pages 58–64