| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1405617 | 1501756 | 2014 | 4 صفحه PDF | دانلود رایگان |
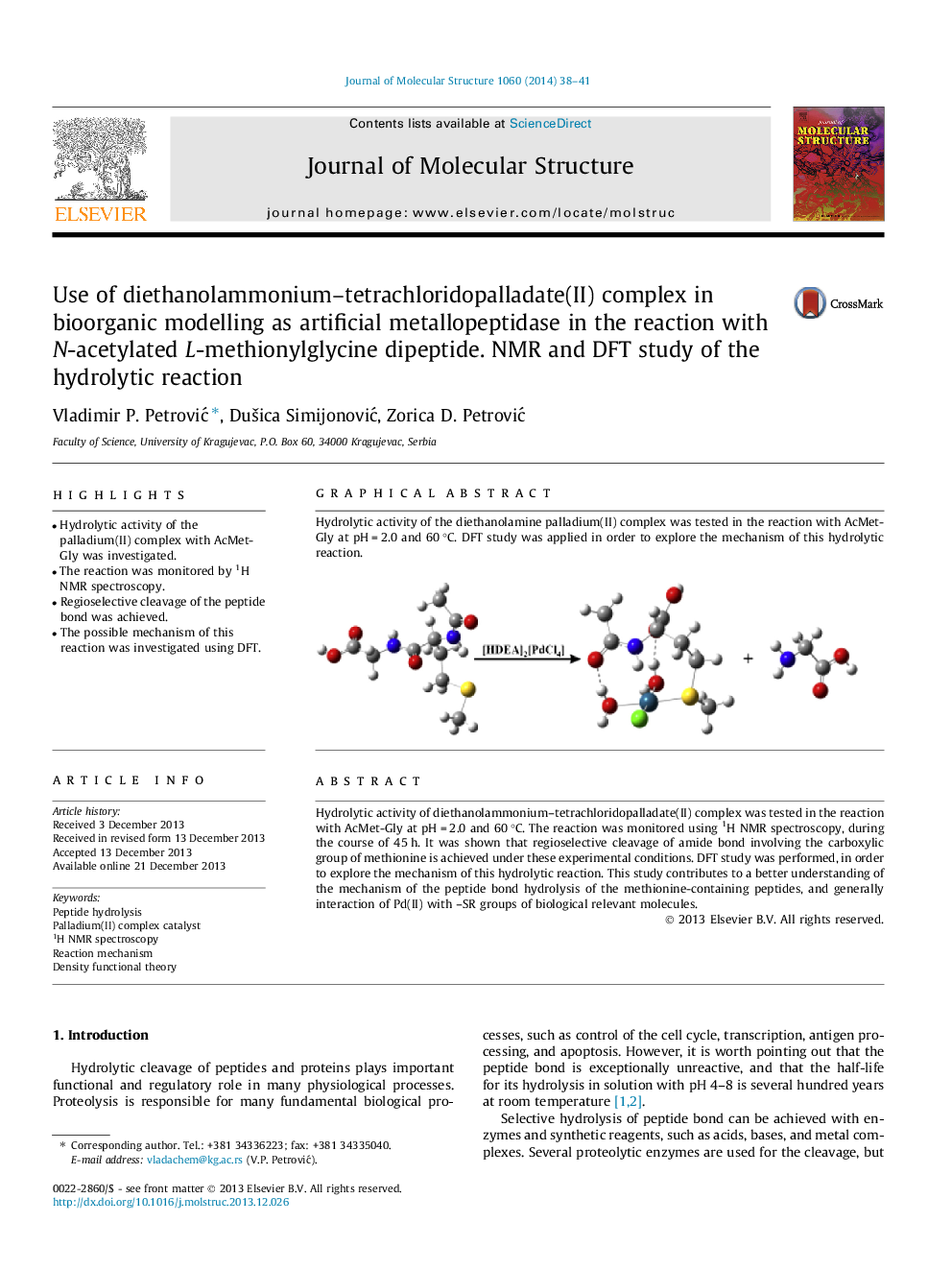
• Hydrolytic activity of the palladium(II) complex with AcMet-Gly was investigated.
• The reaction was monitored by 1H NMR spectroscopy.
• Regioselective cleavage of the peptide bond was achieved.
• The possible mechanism of this reaction was investigated using DFT.
Hydrolytic activity of diethanolammonium–tetrachloridopalladate(II) complex was tested in the reaction with AcMet-Gly at pH = 2.0 and 60 °C. The reaction was monitored using 1H NMR spectroscopy, during the course of 45 h. It was shown that regioselective cleavage of amide bond involving the carboxylic group of methionine is achieved under these experimental conditions. DFT study was performed, in order to explore the mechanism of this hydrolytic reaction. This study contributes to a better understanding of the mechanism of the peptide bond hydrolysis of the methionine-containing peptides, and generally interaction of Pd(II) with –SR groups of biological relevant molecules.
Hydrolytic activity of the diethanolamine palladium(II) complex was tested in the reaction with AcMet-Gly at pH = 2.0 and 60 °C. DFT study was applied in order to explore the mechanism of this hydrolytic reaction.Figure optionsDownload as PowerPoint slide
Journal: Journal of Molecular Structure - Volume 1060, 24 February 2014, Pages 38–41