| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1408128 | 1501723 | 2015 | 9 صفحه PDF | دانلود رایگان |
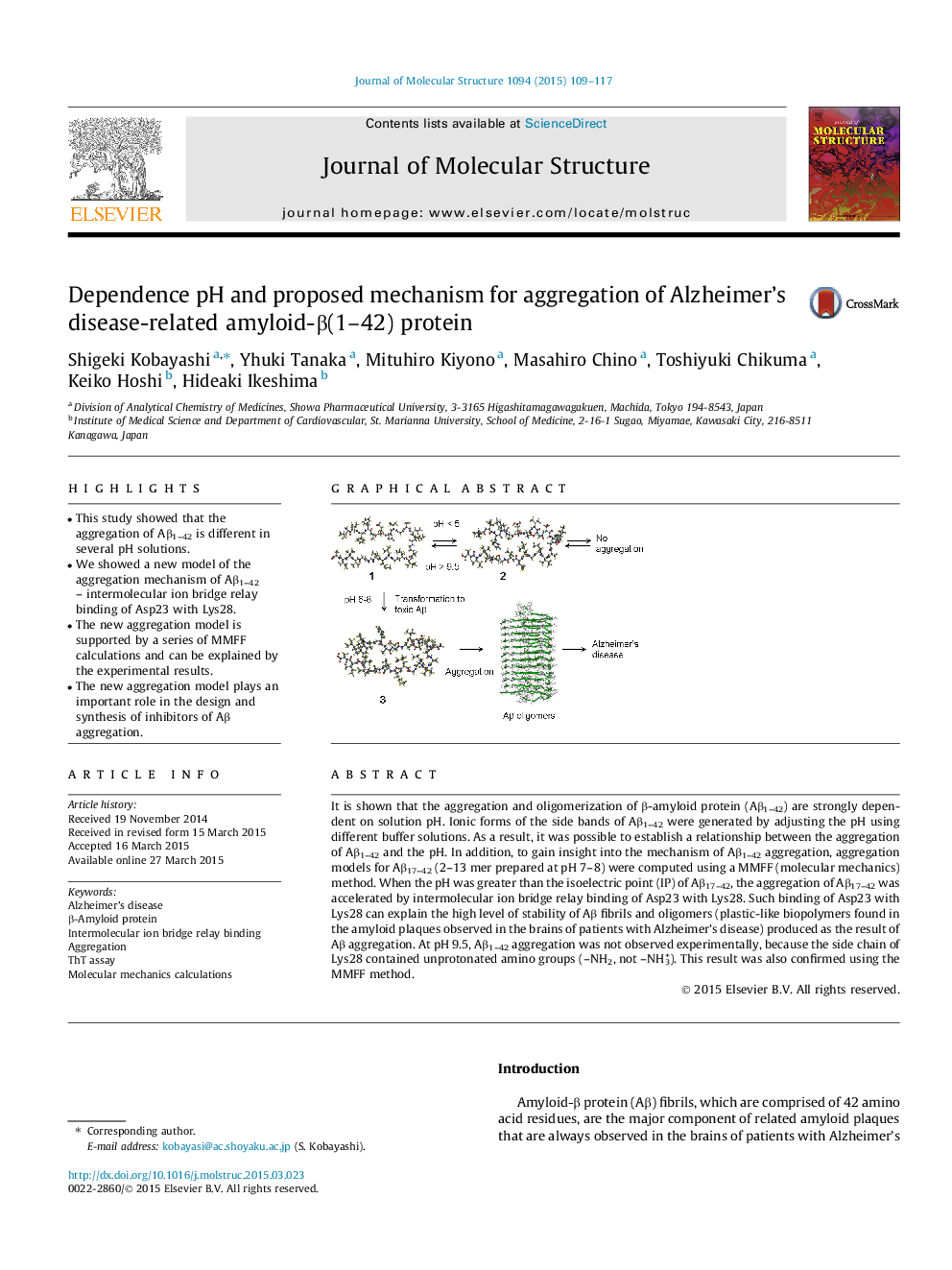
• This study showed that the aggregation of Aβ1–42 is different in several pH solutions.
• We showed a new model of the aggregation mechanism of Aβ1–42 – intermolecular ion bridge relay binding of Asp23 with Lys28.
• The new aggregation model is supported by a series of MMFF calculations and can be explained by the experimental results.
• The new aggregation model plays an important role in the design and synthesis of inhibitors of Aβ aggregation.
It is shown that the aggregation and oligomerization of β-amyloid protein (Aβ1–42) are strongly dependent on solution pH. Ionic forms of the side bands of Aβ1–42 were generated by adjusting the pH using different buffer solutions. As a result, it was possible to establish a relationship between the aggregation of Aβ1–42 and the pH. In addition, to gain insight into the mechanism of Aβ1–42 aggregation, aggregation models for Aβ17–42 (2–13 mer prepared at pH 7–8) were computed using a MMFF (molecular mechanics) method. When the pH was greater than the isoelectric point (IP) of Aβ17–42, the aggregation of Aβ17–42 was accelerated by intermolecular ion bridge relay binding of Asp23 with Lys28. Such binding of Asp23 with Lys28 can explain the high level of stability of Aβ fibrils and oligomers (plastic-like biopolymers found in the amyloid plaques observed in the brains of patients with Alzheimer’s disease) produced as the result of Aβ aggregation. At pH 9.5, Aβ1–42 aggregation was not observed experimentally, because the side chain of Lys28 contained unprotonated amino groups (–NH2, not –NH3+). This result was also confirmed using the MMFF method.
Figure optionsDownload as PowerPoint slide
Journal: Journal of Molecular Structure - Volume 1094, 15 August 2015, Pages 109–117