| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1408130 | 1501723 | 2015 | 7 صفحه PDF | دانلود رایگان |
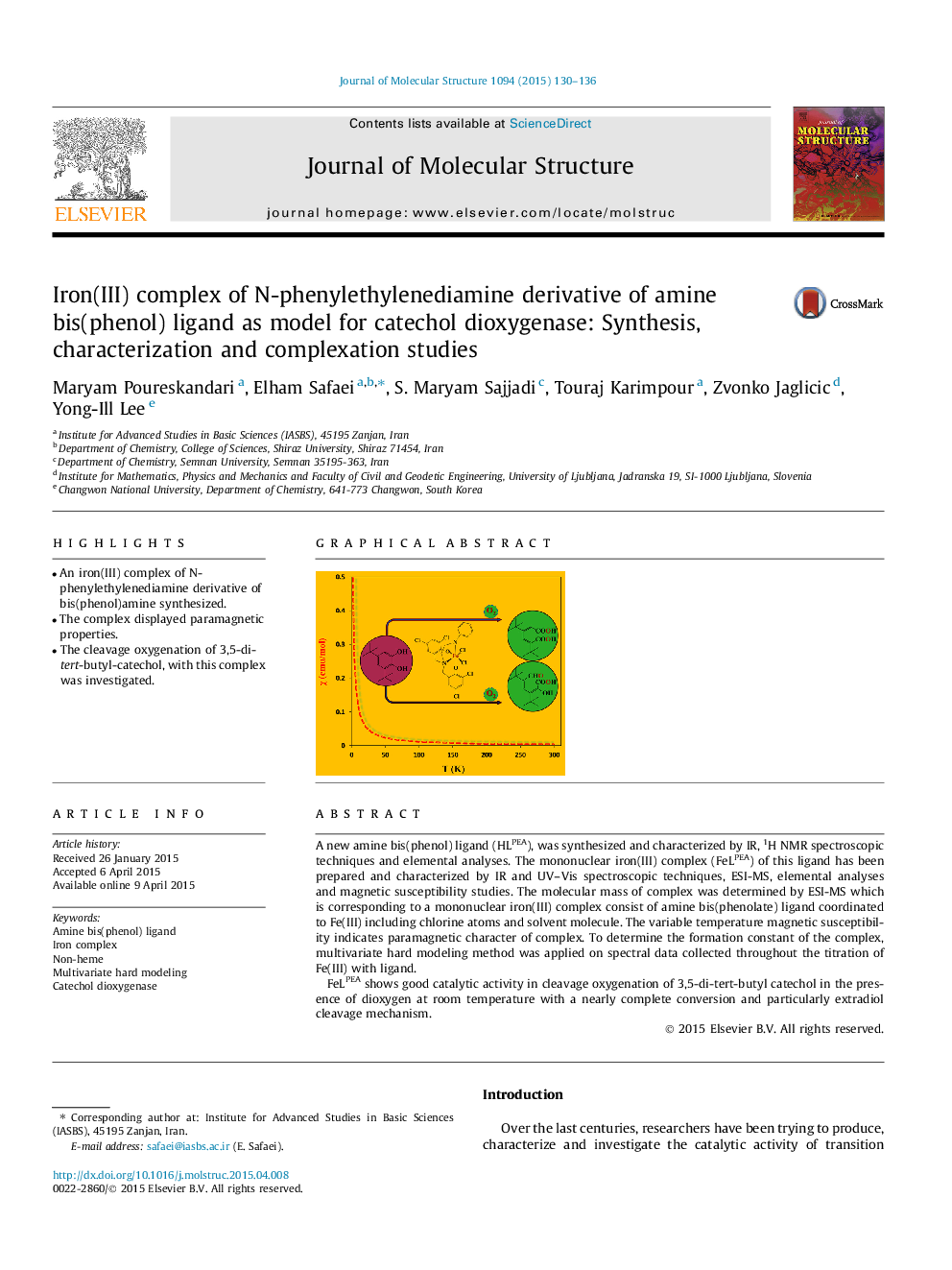
• An iron(III) complex of N-phenylethylenediamine derivative of bis(phenol)amine synthesized.
• The complex displayed paramagnetic properties.
• The cleavage oxygenation of 3,5-di-tert-butyl-catechol, with this complex was investigated.
A new amine bis(phenol) ligand (HLPEA), was synthesized and characterized by IR, 1H NMR spectroscopic techniques and elemental analyses. The mononuclear iron(III) complex (FeLPEA) of this ligand has been prepared and characterized by IR and UV–Vis spectroscopic techniques, ESI-MS, elemental analyses and magnetic susceptibility studies. The molecular mass of complex was determined by ESI-MS which is corresponding to a mononuclear iron(III) complex consist of amine bis(phenolate) ligand coordinated to Fe(III) including chlorine atoms and solvent molecule. The variable temperature magnetic susceptibility indicates paramagnetic character of complex. To determine the formation constant of the complex, multivariate hard modeling method was applied on spectral data collected throughout the titration of Fe(III) with ligand.FeLPEA shows good catalytic activity in cleavage oxygenation of 3,5-di-tert-butyl catechol in the presence of dioxygen at room temperature with a nearly complete conversion and particularly extradiol cleavage mechanism.
Figure optionsDownload as PowerPoint slide
Journal: Journal of Molecular Structure - Volume 1094, 15 August 2015, Pages 130–136