| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1408302 | 1501736 | 2015 | 16 صفحه PDF | دانلود رایگان |
• 2-(4-Bromobenzyl)-5-ethylsulphonyl-1,3-benzoxazole has been synthesized.
• The molecular structure obtained from X-ray single-crystal analysis.
• Density functional modelling studies of the title compound have been reported.
• Experimental and theoretical vibrational spectra are investigated.
• The calculated geometric parameters are in good agreement with the X-ray structure.
Synthesis, crystal structure, Fourier transform infrared spectroscopy (FT-IR) and quantum mechanical studies of the 2-(4-bromobenzyl)-5-ethylsulphonyl-1,3-benzoxazole (C16H14NO3SBr) have been reported. The molecular structure obtained from X-ray single-crystal analysis of the investigated compound in the ground state has been compared using Hartree–Fock (HF) and density functional theory (DFT) with the functional B3LYP and B1B95 using the 6-311++G(d,p) basis set. In addition to the optimized geometrical structures, atomic charges, molecular electrostatic potential (MEP), natural bond orbital (NBO), nonlinear optical (NLO) effects and thermodynamic properties of the compound have been investigated by using DFT. The potential energy surface (PES) scans about four important torsion angles are performed by using B3LYP/6-311++G(d,p) level of theoretical approximation for the compound. The experimental (FT-IR) and calculated vibrational frequencies (using DFT) of the title compound have been compared. The total molecular dipole moment (μ), linear polarisability (α), and the first-order hyperpolarisability (β) were predicted by using DFT with different basis sets 6-31G(d), 6-31+G(d,p), 6-31++G(d,p), 6-311+G(d) and 6-311++G(d,p) for investigating the effects of basis sets on the NLO properties. Our computational results yield that βtot for the title compound is greater than those of urea. The standard thermodynamic functions were obtained for the title compound with the temperature ranging from 200 to 450 K.
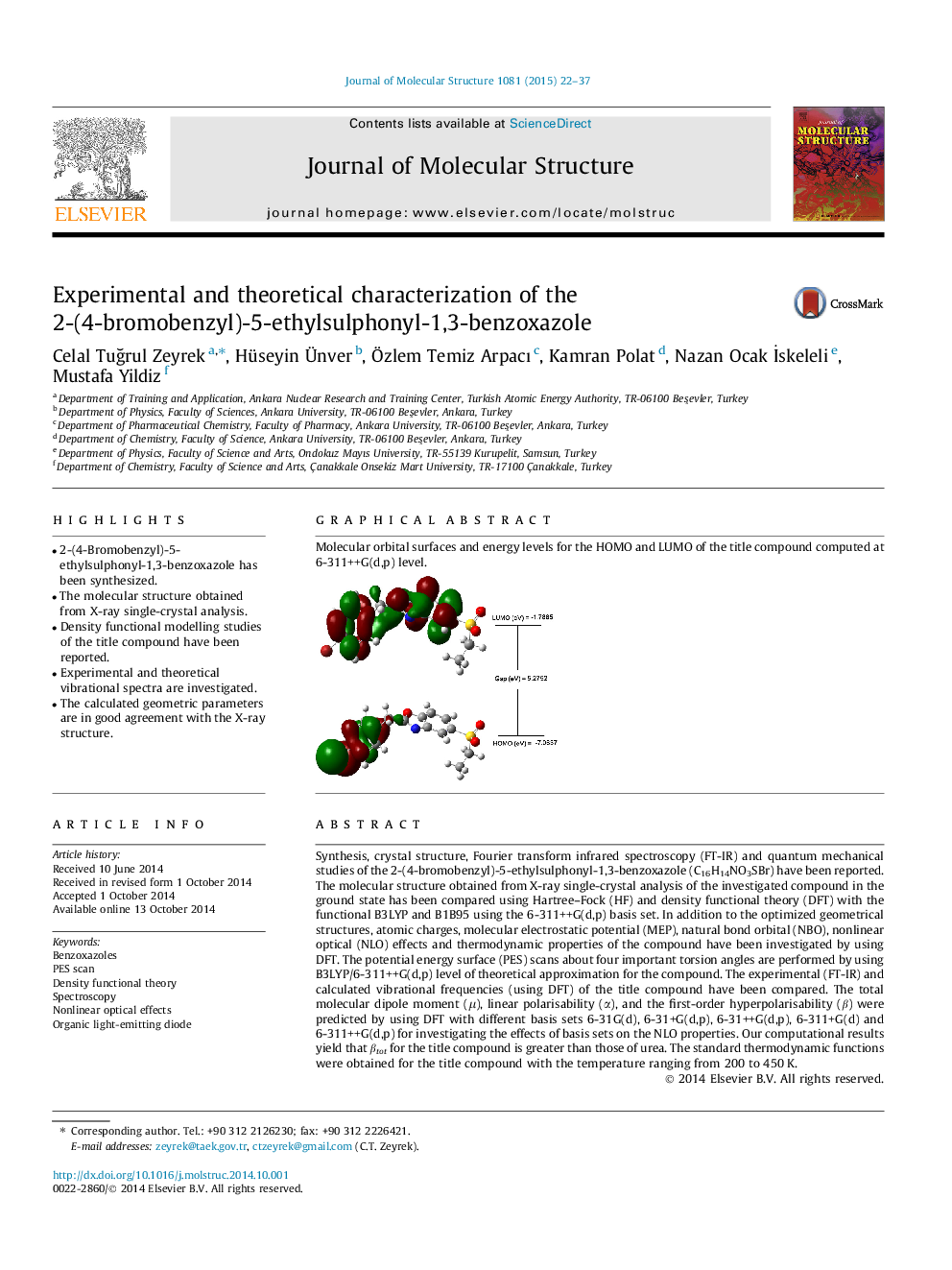
Molecular orbital surfaces and energy levels for the HOMO and LUMO of the title compound computed at 6-311++G(d,p) level.Figure optionsDownload as PowerPoint slide
Journal: Journal of Molecular Structure - Volume 1081, 5 February 2015, Pages 22–37