| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1408450 | 1501741 | 2014 | 10 صفحه PDF | دانلود رایگان |
• IHB strength of TFDMHD has been estimated by using AIM and NBO method.
• Complete analysis of vibrational spectra and DFT investigations performed.
• TFDMHD’s IHB is stronger than that of DMHD and weaker than that of TFAA.
• Experimental results indicate coexisting of three stable conformers in the sample.
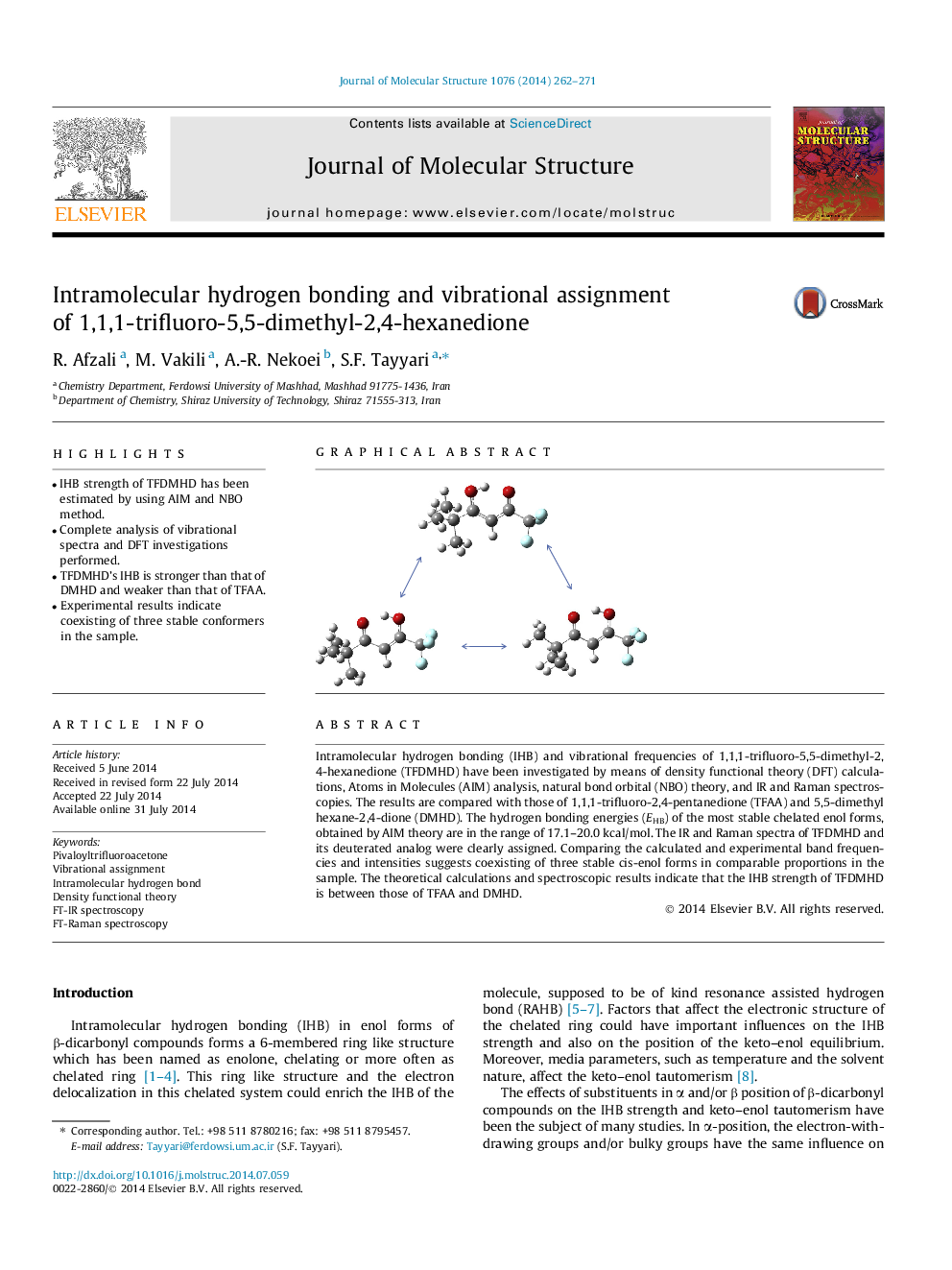
Intramolecular hydrogen bonding (IHB) and vibrational frequencies of 1,1,1-trifluoro-5,5-dimethyl-2,4-hexanedione (TFDMHD) have been investigated by means of density functional theory (DFT) calculations, Atoms in Molecules (AIM) analysis, natural bond orbital (NBO) theory, and IR and Raman spectroscopies. The results are compared with those of 1,1,1-trifluoro-2,4-pentanedione (TFAA) and 5,5-dimethyl hexane-2,4-dione (DMHD). The hydrogen bonding energies (EHB) of the most stable chelated enol forms, obtained by AIM theory are in the range of 17.1–20.0 kcal/mol. The IR and Raman spectra of TFDMHD and its deuterated analog were clearly assigned. Comparing the calculated and experimental band frequencies and intensities suggests coexisting of three stable cis-enol forms in comparable proportions in the sample. The theoretical calculations and spectroscopic results indicate that the IHB strength of TFDMHD is between those of TFAA and DMHD.
Figure optionsDownload as PowerPoint slide
Journal: Journal of Molecular Structure - Volume 1076, 5 November 2014, Pages 262–271