| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1408466 | 1501741 | 2014 | 9 صفحه PDF | دانلود رایگان |
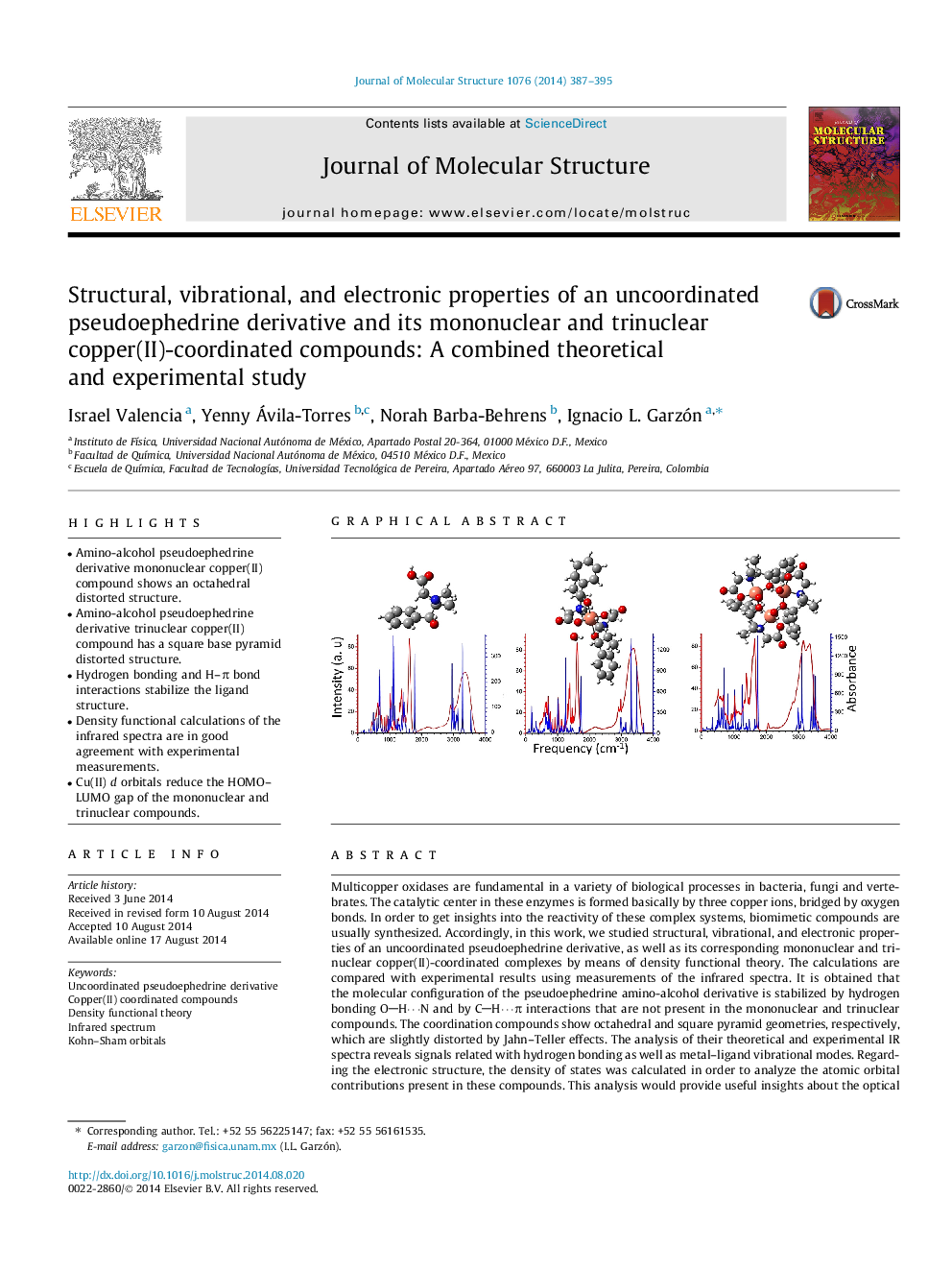
• Amino-alcohol pseudoephedrine derivative mononuclear copper(II) compound shows an octahedral distorted structure.
• Amino-alcohol pseudoephedrine derivative trinuclear copper(II) compound has a square base pyramid distorted structure.
• Hydrogen bonding and H–π bond interactions stabilize the ligand structure.
• Density functional calculations of the infrared spectra are in good agreement with experimental measurements.
• Cu(II) d orbitals reduce the HOMO–LUMO gap of the mononuclear and trinuclear compounds.
Multicopper oxidases are fundamental in a variety of biological processes in bacteria, fungi and vertebrates. The catalytic center in these enzymes is formed basically by three copper ions, bridged by oxygen bonds. In order to get insights into the reactivity of these complex systems, biomimetic compounds are usually synthesized. Accordingly, in this work, we studied structural, vibrational, and electronic properties of an uncoordinated pseudoephedrine derivative, as well as its corresponding mononuclear and trinuclear copper(II)-coordinated complexes by means of density functional theory. The calculations are compared with experimental results using measurements of the infrared spectra. It is obtained that the molecular configuration of the pseudoephedrine amino-alcohol derivative is stabilized by hydrogen bonding OH⋯N and by CH⋯π interactions that are not present in the mononuclear and trinuclear compounds. The coordination compounds show octahedral and square pyramid geometries, respectively, which are slightly distorted by Jahn–Teller effects. The analysis of their theoretical and experimental IR spectra reveals signals related with hydrogen bonding as well as metal–ligand vibrational modes. Regarding the electronic structure, the density of states was calculated in order to analyze the atomic orbital contributions present in these compounds. This analysis would provide useful insights about the optical behavior, for example, in the visible region of the spectrum of the coordinated compounds. At these energies, the optical absorption would be influenced by the orbital interaction of the Cu2+d orbitals with sp ones of the ligand, reflecting a decrease of the HOMO–LUMO gap of the organic ligand due to the presence of the copper(II) ions.
Figure optionsDownload as PowerPoint slide
Journal: Journal of Molecular Structure - Volume 1076, 5 November 2014, Pages 387–395