| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1409338 | 1501751 | 2014 | 12 صفحه PDF | دانلود رایگان |
• A charge transfer or proton transfer complex is synthesized and confirmed by spectral investigations.
• Spectrophotometric studies were made in different polar solvents.
• Benesi–Hildebrand equation in various solvents was used to determine KCT, εCT, and ECT of the CT complex.
• Single-crystal X-ray studies confirmed the H-bonded network.
• Thermal analysis (TGA–DTA) was also used to confirm the thermal fragmentation and the stability of the CT complex.
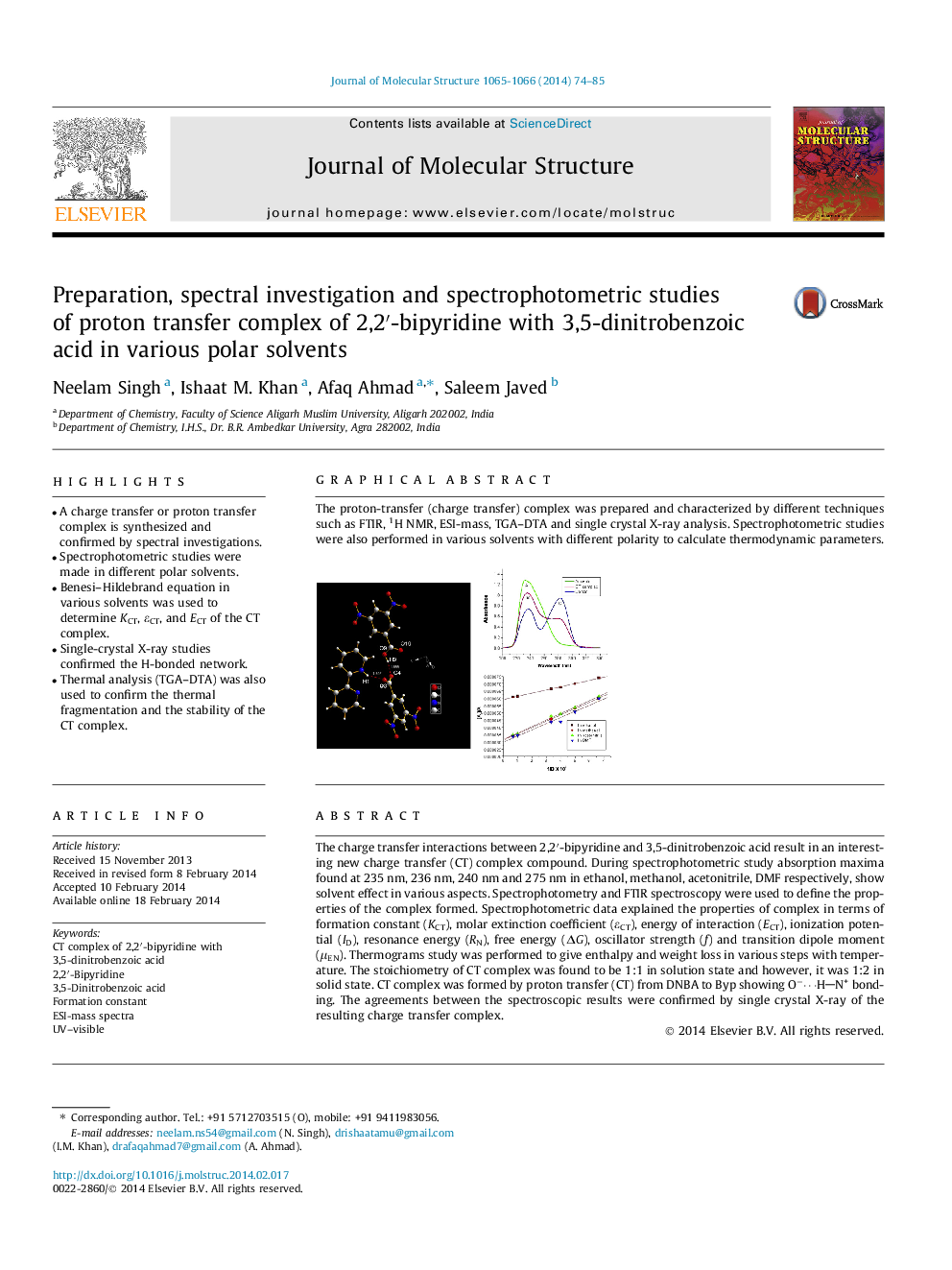
The charge transfer interactions between 2,2′-bipyridine and 3,5-dinitrobenzoic acid result in an interesting new charge transfer (CT) complex compound. During spectrophotometric study absorption maxima found at 235 nm, 236 nm, 240 nm and 275 nm in ethanol, methanol, acetonitrile, DMF respectively, show solvent effect in various aspects. Spectrophotometry and FTIR spectroscopy were used to define the properties of the complex formed. Spectrophotometric data explained the properties of complex in terms of formation constant (KCT), molar extinction coefficient (εCT), energy of interaction (ECT), ionization potential (ID), resonance energy (RN), free energy (ΔG), oscillator strength (f) and transition dipole moment (μEN). Thermograms study was performed to give enthalpy and weight loss in various steps with temperature. The stoichiometry of CT complex was found to be 1:1 in solution state and however, it was 1:2 in solid state. CT complex was formed by proton transfer (CT) from DNBA to Byp showing O−⋯HN+ bonding. The agreements between the spectroscopic results were confirmed by single crystal X-ray of the resulting charge transfer complex.
The proton-transfer (charge transfer) complex was prepared and characterized by different techniques such as FTIR, 1H NMR, ESI-mass, TGA–DTA and single crystal X-ray analysis. Spectrophotometric studies were also performed in various solvents with different polarity to calculate thermodynamic parameters.Figure optionsDownload as PowerPoint slide
Journal: Journal of Molecular Structure - Volumes 1065–1066, 22 May 2014, Pages 74–85