| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 145311 | 456338 | 2016 | 10 صفحه PDF | دانلود رایگان |
• Bentonite decreased the aggregation of nZVI particles and increased their reactivity.
• Persulfate had a negligible effect on Cr(VI) reduction in B-nZVI/PS system.
• A synergistic effect between Cr(VI) reduction and phenol oxidation was achieved.
• A positive correlation between persulfate decomposition and Fe2+ was found.
• The intermediate products of both Cr(VI) and phenol were identified.
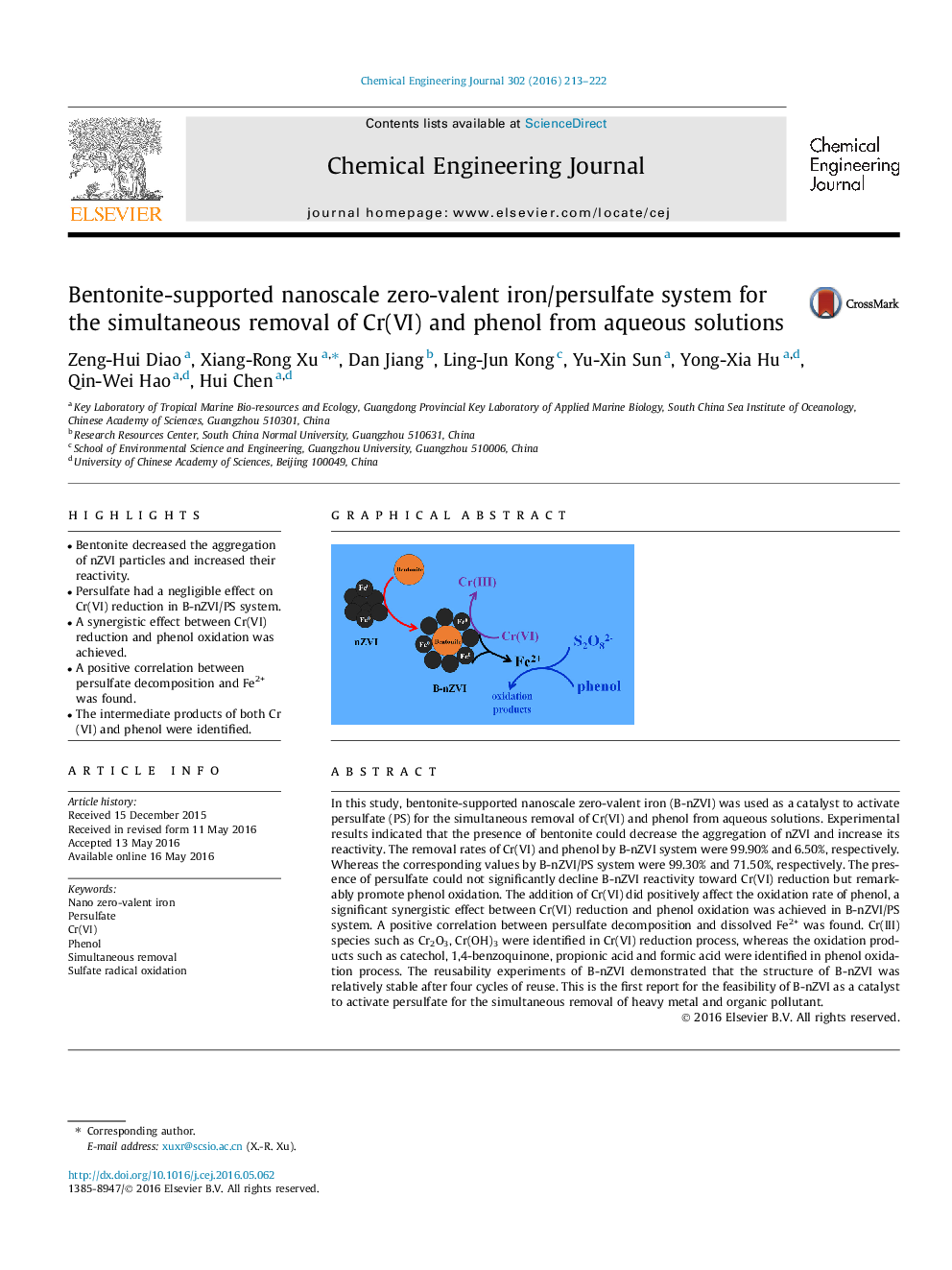
In this study, bentonite-supported nanoscale zero-valent iron (B-nZVI) was used as a catalyst to activate persulfate (PS) for the simultaneous removal of Cr(VI) and phenol from aqueous solutions. Experimental results indicated that the presence of bentonite could decrease the aggregation of nZVI and increase its reactivity. The removal rates of Cr(VI) and phenol by B-nZVI system were 99.90% and 6.50%, respectively. Whereas the corresponding values by B-nZVI/PS system were 99.30% and 71.50%, respectively. The presence of persulfate could not significantly decline B-nZVI reactivity toward Cr(VI) reduction but remarkably promote phenol oxidation. The addition of Cr(VI) did positively affect the oxidation rate of phenol, a significant synergistic effect between Cr(VI) reduction and phenol oxidation was achieved in B-nZVI/PS system. A positive correlation between persulfate decomposition and dissolved Fe2+ was found. Cr(III) species such as Cr2O3, Cr(OH)3 were identified in Cr(VI) reduction process, whereas the oxidation products such as catechol, 1,4-benzoquinone, propionic acid and formic acid were identified in phenol oxidation process. The reusability experiments of B-nZVI demonstrated that the structure of B-nZVI was relatively stable after four cycles of reuse. This is the first report for the feasibility of B-nZVI as a catalyst to activate persulfate for the simultaneous removal of heavy metal and organic pollutant.
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 302, 15 October 2016, Pages 213–222