| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 145619 | 456345 | 2016 | 11 صفحه PDF | دانلود رایگان |
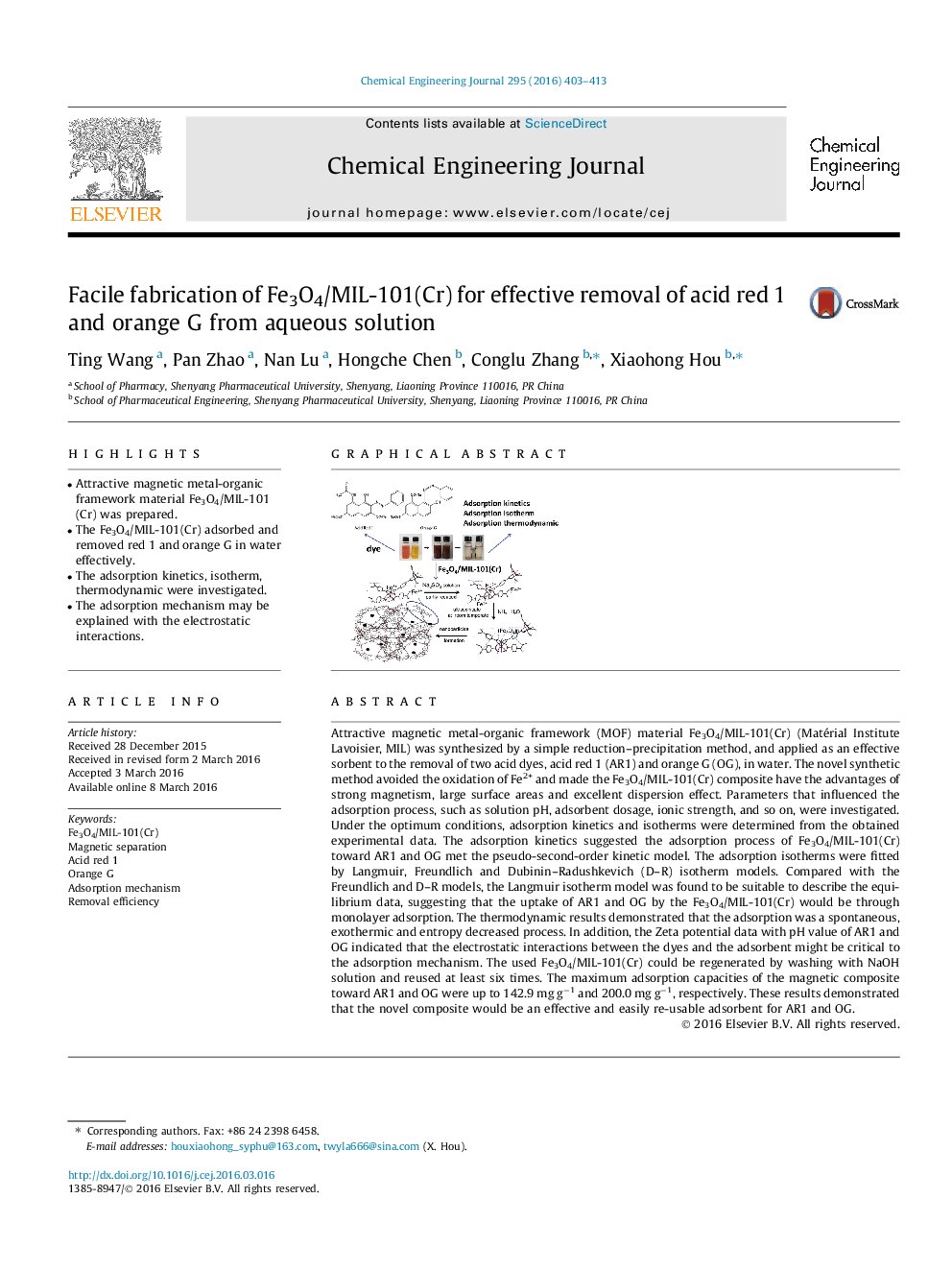
• Attractive magnetic metal-organic framework material Fe3O4/MIL-101(Cr) was prepared.
• The Fe3O4/MIL-101(Cr) adsorbed and removed red 1 and orange G in water effectively.
• The adsorption kinetics, isotherm, thermodynamic were investigated.
• The adsorption mechanism may be explained with the electrostatic interactions.
Attractive magnetic metal-organic framework (MOF) material Fe3O4/MIL-101(Cr) (Matérial Institute Lavoisier, MIL) was synthesized by a simple reduction–precipitation method, and applied as an effective sorbent to the removal of two acid dyes, acid red 1 (AR1) and orange G (OG), in water. The novel synthetic method avoided the oxidation of Fe2+ and made the Fe3O4/MIL-101(Cr) composite have the advantages of strong magnetism, large surface areas and excellent dispersion effect. Parameters that influenced the adsorption process, such as solution pH, adsorbent dosage, ionic strength, and so on, were investigated. Under the optimum conditions, adsorption kinetics and isotherms were determined from the obtained experimental data. The adsorption kinetics suggested the adsorption process of Fe3O4/MIL-101(Cr) toward AR1 and OG met the pseudo-second-order kinetic model. The adsorption isotherms were fitted by Langmuir, Freundlich and Dubinin–Radushkevich (D–R) isotherm models. Compared with the Freundlich and D–R models, the Langmuir isotherm model was found to be suitable to describe the equilibrium data, suggesting that the uptake of AR1 and OG by the Fe3O4/MIL-101(Cr) would be through monolayer adsorption. The thermodynamic results demonstrated that the adsorption was a spontaneous, exothermic and entropy decreased process. In addition, the Zeta potential data with pH value of AR1 and OG indicated that the electrostatic interactions between the dyes and the adsorbent might be critical to the adsorption mechanism. The used Fe3O4/MIL-101(Cr) could be regenerated by washing with NaOH solution and reused at least six times. The maximum adsorption capacities of the magnetic composite toward AR1 and OG were up to 142.9 mg g−1 and 200.0 mg g−1, respectively. These results demonstrated that the novel composite would be an effective and easily re-usable adsorbent for AR1 and OG.
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 295, 1 July 2016, Pages 403–413