| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 145656 | 456347 | 2016 | 10 صفحه PDF | دانلود رایگان |
• Fe-modified θ-Al2O3 supports were prepared using γ-Al2O3, Fe nitrate or oxalate.
• Au0 nanoparticles (<3 nm) were deposited on the Fe-modified θ-Al2O3 supports.
• 1%Au/θ-Al2−xFexO3 catalysts are highly effective in CO oxidation in the presence of NH3.
• Fe3+ introduction using nitrate suppresses the NH3 oxidation over the gold catalyst.
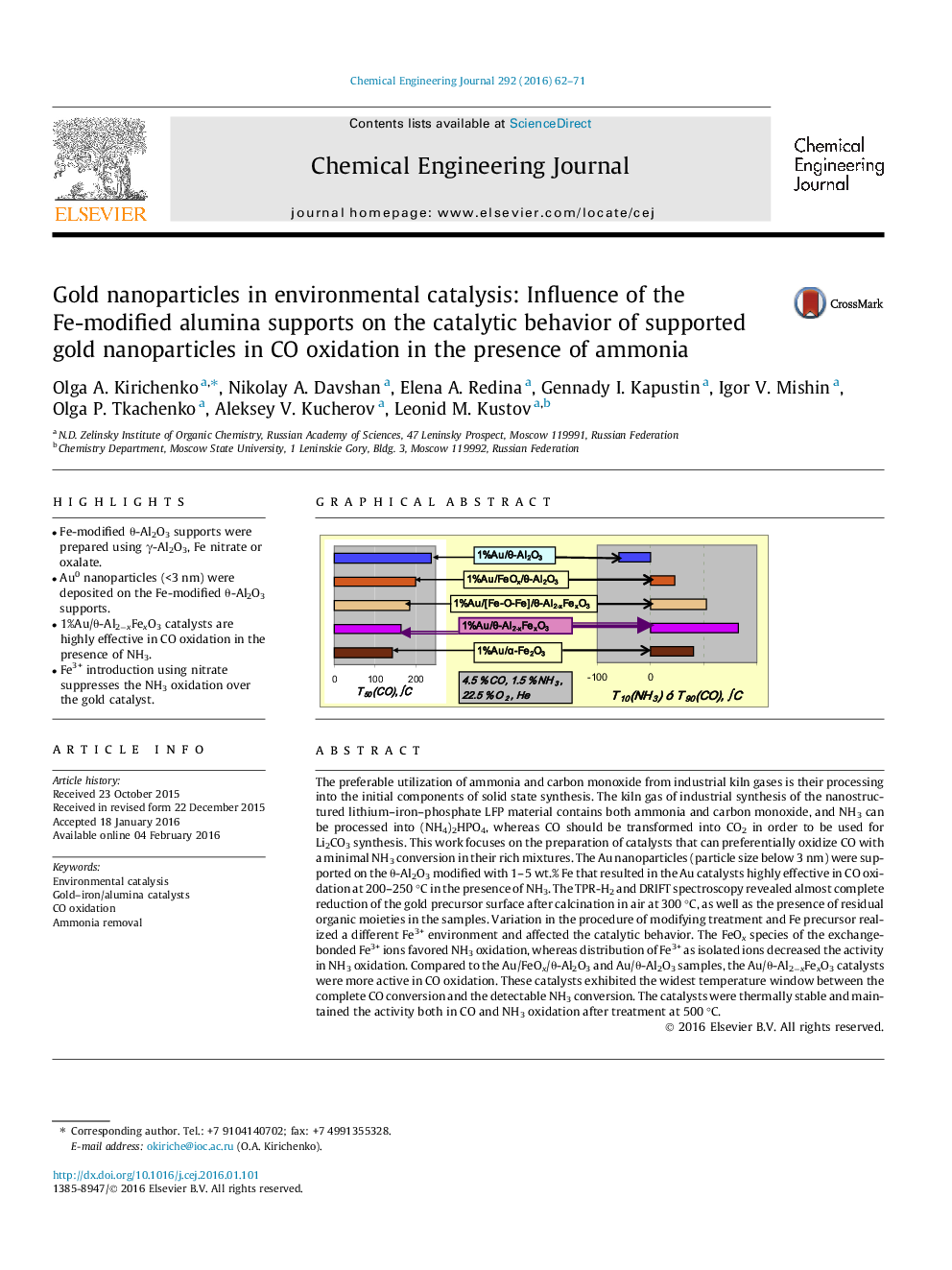
The preferable utilization of ammonia and carbon monoxide from industrial kiln gases is their processing into the initial components of solid state synthesis. The kiln gas of industrial synthesis of the nanostructured lithium–iron–phosphate LFP material contains both ammonia and carbon monoxide, and NH3 can be processed into (NH4)2HPO4, whereas CO should be transformed into CO2 in order to be used for Li2CO3 synthesis. This work focuses on the preparation of catalysts that can preferentially oxidize CO with a minimal NH3 conversion in their rich mixtures. The Au nanoparticles (particle size below 3 nm) were supported on the θ-Al2O3 modified with 1–5 wt.% Fe that resulted in the Au catalysts highly effective in CO oxidation at 200–250 °C in the presence of NH3. The TPR-H2 and DRIFT spectroscopy revealed almost complete reduction of the gold precursor surface after calcination in air at 300 °C, as well as the presence of residual organic moieties in the samples. Variation in the procedure of modifying treatment and Fe precursor realized a different Fe3+ environment and affected the catalytic behavior. The FeOx species of the exchange-bonded Fe3+ ions favored NH3 oxidation, whereas distribution of Fe3+ as isolated ions decreased the activity in NH3 oxidation. Compared to the Au/FeOx/θ-Al2O3 and Au/θ-Al2O3 samples, the Au/θ-Al2−xFexO3 catalysts were more active in CO oxidation. These catalysts exhibited the widest temperature window between the complete CO conversion and the detectable NH3 conversion. The catalysts were thermally stable and maintained the activity both in CO and NH3 oxidation after treatment at 500 °C.
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 292, 15 May 2016, Pages 62–71