| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 146194 | 456363 | 2015 | 14 صفحه PDF | دانلود رایگان |
• Detailed kinetic study of NMP of styrene mediated by BlocBuilder MA (MAMA-SG1).
• NMP kinetic model accounting for chain transfer and diffusional limitations.
• Estimation of NMP (de)activation Arrhenius parameters.
• Kinetic Monte Carlo visualization of polymer chains according to chain initiation.
• Model-based optimization via a stepwise temperature program.
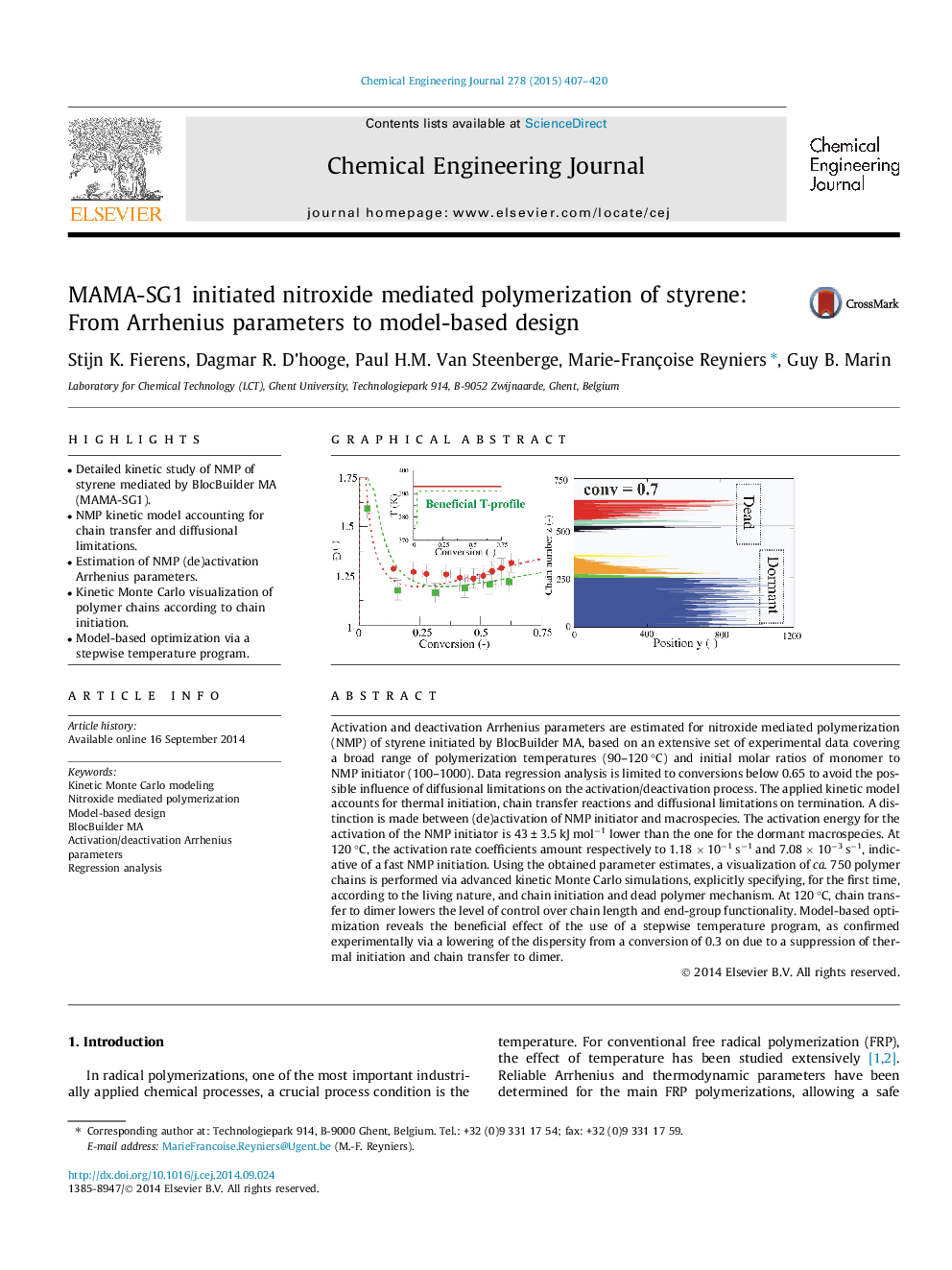
Activation and deactivation Arrhenius parameters are estimated for nitroxide mediated polymerization (NMP) of styrene initiated by BlocBuilder MA, based on an extensive set of experimental data covering a broad range of polymerization temperatures (90–120 °C) and initial molar ratios of monomer to NMP initiator (100–1000). Data regression analysis is limited to conversions below 0.65 to avoid the possible influence of diffusional limitations on the activation/deactivation process. The applied kinetic model accounts for thermal initiation, chain transfer reactions and diffusional limitations on termination. A distinction is made between (de)activation of NMP initiator and macrospecies. The activation energy for the activation of the NMP initiator is 43 ± 3.5 kJ mol−1 lower than the one for the dormant macrospecies. At 120 °C, the activation rate coefficients amount respectively to 1.18 × 10−1 s−1 and 7.08 × 10−3 s−1, indicative of a fast NMP initiation. Using the obtained parameter estimates, a visualization of ca. 750 polymer chains is performed via advanced kinetic Monte Carlo simulations, explicitly specifying, for the first time, according to the living nature, and chain initiation and dead polymer mechanism. At 120 °C, chain transfer to dimer lowers the level of control over chain length and end-group functionality. Model-based optimization reveals the beneficial effect of the use of a stepwise temperature program, as confirmed experimentally via a lowering of the dispersity from a conversion of 0.3 on due to a suppression of thermal initiation and chain transfer to dimer.
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 278, 15 October 2015, Pages 407–420