| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 146451 | 456371 | 2015 | 10 صفحه PDF | دانلود رایگان |
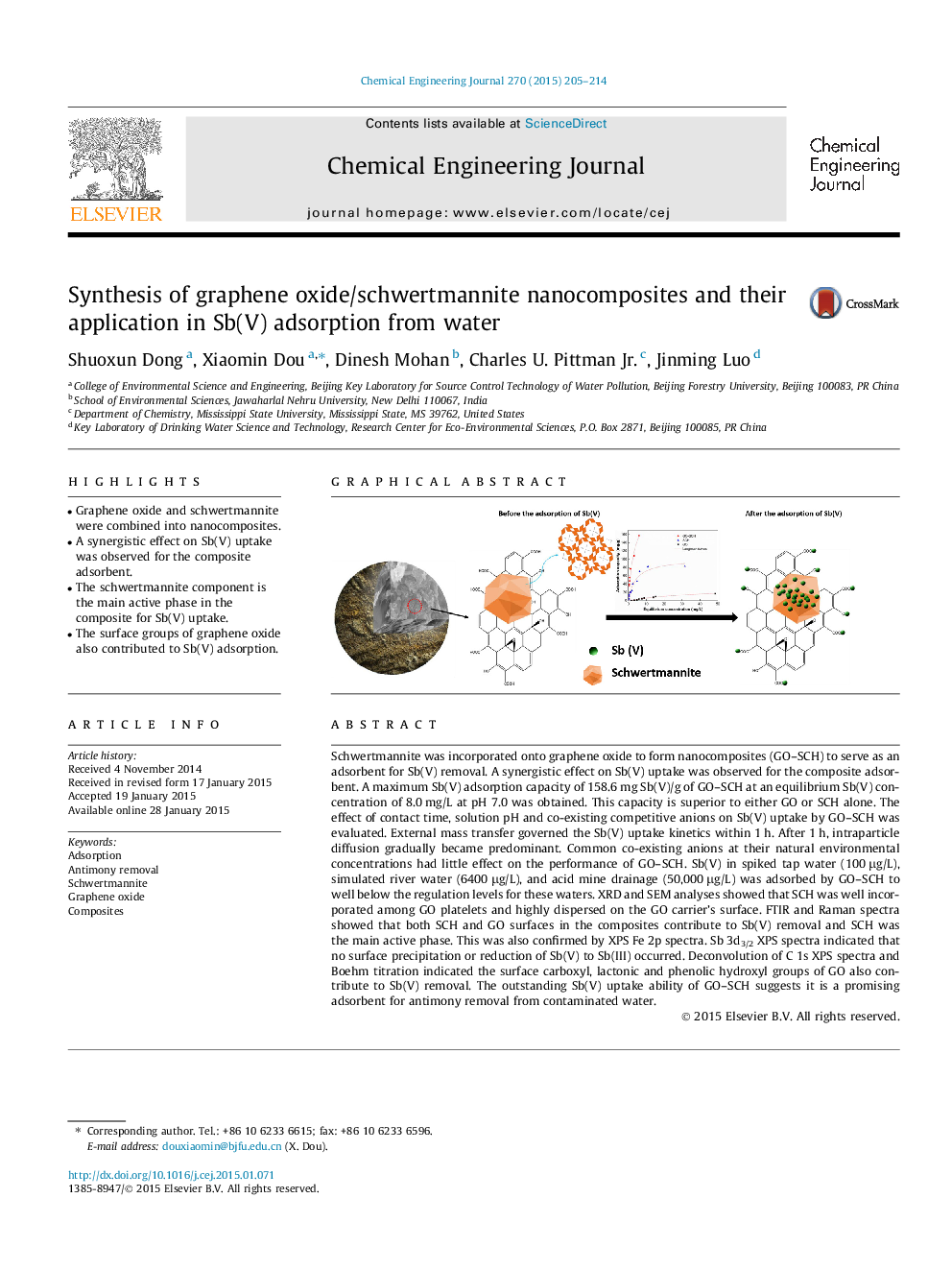
• Graphene oxide and schwertmannite were combined into nanocomposites.
• A synergistic effect on Sb(V) uptake was observed for the composite adsorbent.
• The schwertmannite component is the main active phase in the composite for Sb(V) uptake.
• The surface groups of graphene oxide also contributed to Sb(V) adsorption.
Schwertmannite was incorporated onto graphene oxide to form nanocomposites (GO–SCH) to serve as an adsorbent for Sb(V) removal. A synergistic effect on Sb(V) uptake was observed for the composite adsorbent. A maximum Sb(V) adsorption capacity of 158.6 mg Sb(V)/g of GO–SCH at an equilibrium Sb(V) concentration of 8.0 mg/L at pH 7.0 was obtained. This capacity is superior to either GO or SCH alone. The effect of contact time, solution pH and co-existing competitive anions on Sb(V) uptake by GO–SCH was evaluated. External mass transfer governed the Sb(V) uptake kinetics within 1 h. After 1 h, intraparticle diffusion gradually became predominant. Common co-existing anions at their natural environmental concentrations had little effect on the performance of GO–SCH. Sb(V) in spiked tap water (100 μg/L), simulated river water (6400 μg/L), and acid mine drainage (50,000 μg/L) was adsorbed by GO–SCH to well below the regulation levels for these waters. XRD and SEM analyses showed that SCH was well incorporated among GO platelets and highly dispersed on the GO carrier’s surface. FTIR and Raman spectra showed that both SCH and GO surfaces in the composites contribute to Sb(V) removal and SCH was the main active phase. This was also confirmed by XPS Fe 2p spectra. Sb 3d3/2 XPS spectra indicated that no surface precipitation or reduction of Sb(V) to Sb(III) occurred. Deconvolution of C 1s XPS spectra and Boehm titration indicated the surface carboxyl, lactonic and phenolic hydroxyl groups of GO also contribute to Sb(V) removal. The outstanding Sb(V) uptake ability of GO–SCH suggests it is a promising adsorbent for antimony removal from contaminated water.
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 270, 15 June 2015, Pages 205–214