| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 147118 | 456385 | 2014 | 6 صفحه PDF | دانلود رایگان |
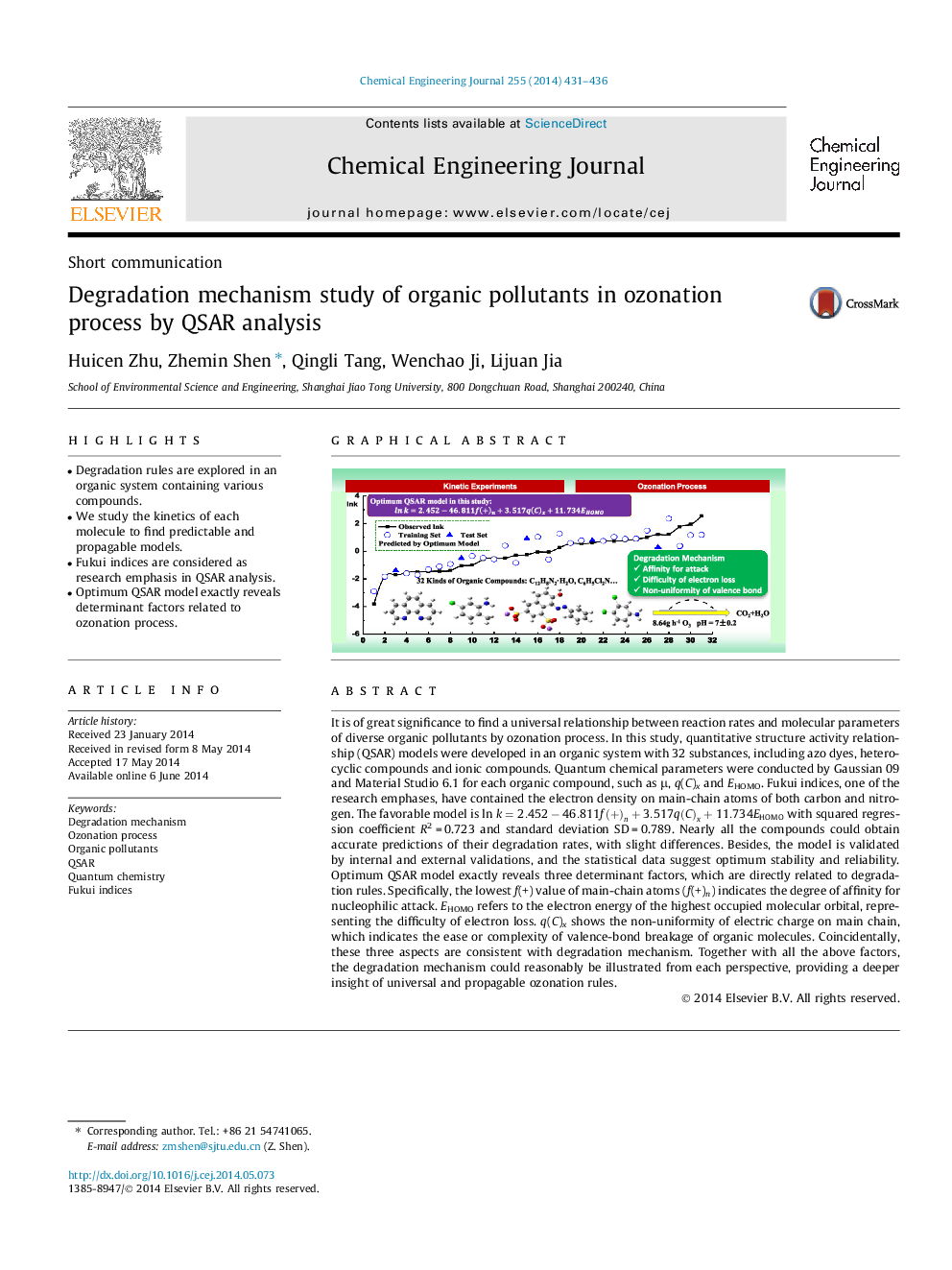
• Degradation rules are explored in an organic system containing various compounds.
• We study the kinetics of each molecule to find predictable and propagable models.
• Fukui indices are considered as research emphasis in QSAR analysis.
• Optimum QSAR model exactly reveals determinant factors related to ozonation process.
It is of great significance to find a universal relationship between reaction rates and molecular parameters of diverse organic pollutants by ozonation process. In this study, quantitative structure activity relationship (QSAR) models were developed in an organic system with 32 substances, including azo dyes, heterocyclic compounds and ionic compounds. Quantum chemical parameters were conducted by Gaussian 09 and Material Studio 6.1 for each organic compound, such as μ, q(C)x and EHOMO. Fukui indices, one of the research emphases, have contained the electron density on main-chain atoms of both carbon and nitrogen. The favorable model is lnk=2.452-46.811f(+)n+3.517q(C)x+11.734EHOMO with squared regression coefficient R2 = 0.723 and standard deviation SD = 0.789. Nearly all the compounds could obtain accurate predictions of their degradation rates, with slight differences. Besides, the model is validated by internal and external validations, and the statistical data suggest optimum stability and reliability. Optimum QSAR model exactly reveals three determinant factors, which are directly related to degradation rules. Specifically, the lowest f(+) value of main-chain atoms (f(+)n) indicates the degree of affinity for nucleophilic attack. EHOMO refers to the electron energy of the highest occupied molecular orbital, representing the difficulty of electron loss. q(C)x shows the non-uniformity of electric charge on main chain, which indicates the ease or complexity of valence-bond breakage of organic molecules. Coincidentally, these three aspects are consistent with degradation mechanism. Together with all the above factors, the degradation mechanism could reasonably be illustrated from each perspective, providing a deeper insight of universal and propagable ozonation rules.
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 255, 1 November 2014, Pages 431–436