| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 147354 | 456390 | 2014 | 11 صفحه PDF | دانلود رایگان |
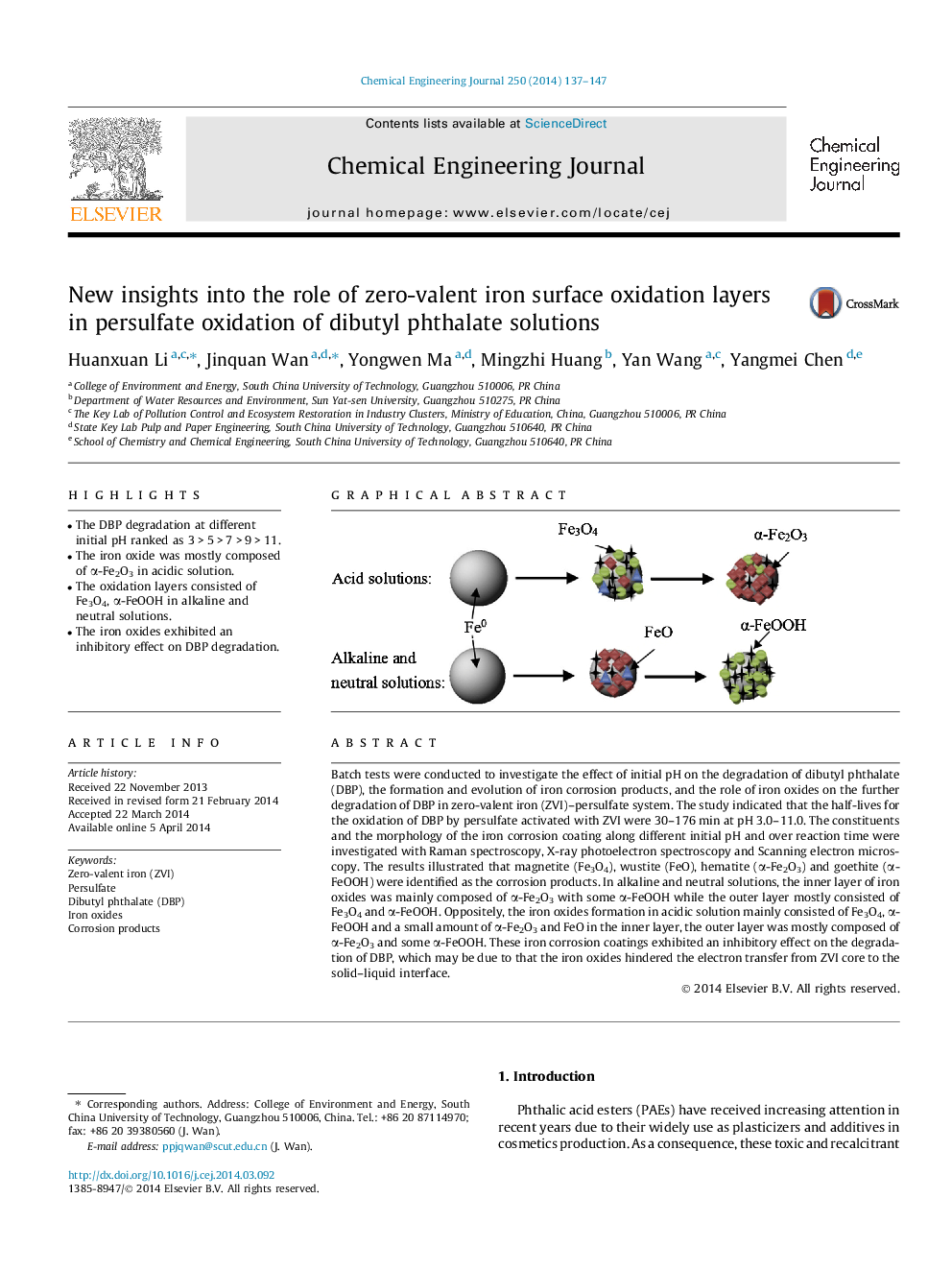
• The DBP degradation at different initial pH ranked as 3 > 5 > 7 > 9 > 11.
• The iron oxide was mostly composed of α-Fe2O3 in acidic solution.
• The oxidation layers consisted of Fe3O4, α-FeOOH in alkaline and neutral solutions.
• The iron oxides exhibited an inhibitory effect on DBP degradation.
Batch tests were conducted to investigate the effect of initial pH on the degradation of dibutyl phthalate (DBP), the formation and evolution of iron corrosion products, and the role of iron oxides on the further degradation of DBP in zero-valent iron (ZVI)–persulfate system. The study indicated that the half-lives for the oxidation of DBP by persulfate activated with ZVI were 30–176 min at pH 3.0–11.0. The constituents and the morphology of the iron corrosion coating along different initial pH and over reaction time were investigated with Raman spectroscopy, X-ray photoelectron spectroscopy and Scanning electron microscopy. The results illustrated that magnetite (Fe3O4), wustite (FeO), hematite (α-Fe2O3) and goethite (α-FeOOH) were identified as the corrosion products. In alkaline and neutral solutions, the inner layer of iron oxides was mainly composed of α-Fe2O3 with some α-FeOOH while the outer layer mostly consisted of Fe3O4 and α-FeOOH. Oppositely, the iron oxides formation in acidic solution mainly consisted of Fe3O4, α-FeOOH and a small amount of α-Fe2O3 and FeO in the inner layer, the outer layer was mostly composed of α-Fe2O3 and some α-FeOOH. These iron corrosion coatings exhibited an inhibitory effect on the degradation of DBP, which may be due to that the iron oxides hindered the electron transfer from ZVI core to the solid–liquid interface.
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 250, 15 August 2014, Pages 137–147