| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 147683 | 456398 | 2014 | 9 صفحه PDF | دانلود رایگان |
• Magnetic and dual conductive imprinted photocatalysts (MCIPs) were prepared.
• The MCIPs were used to treat 1-methylimidazole-2-thiol.
• The MCIPs exhibited high photocatalytic activity and selectivity.
• The photodegradation processes obeyed the pseudo-first-order kinetic reaction.
• The mechanism of photodegradation were carefully discussed.
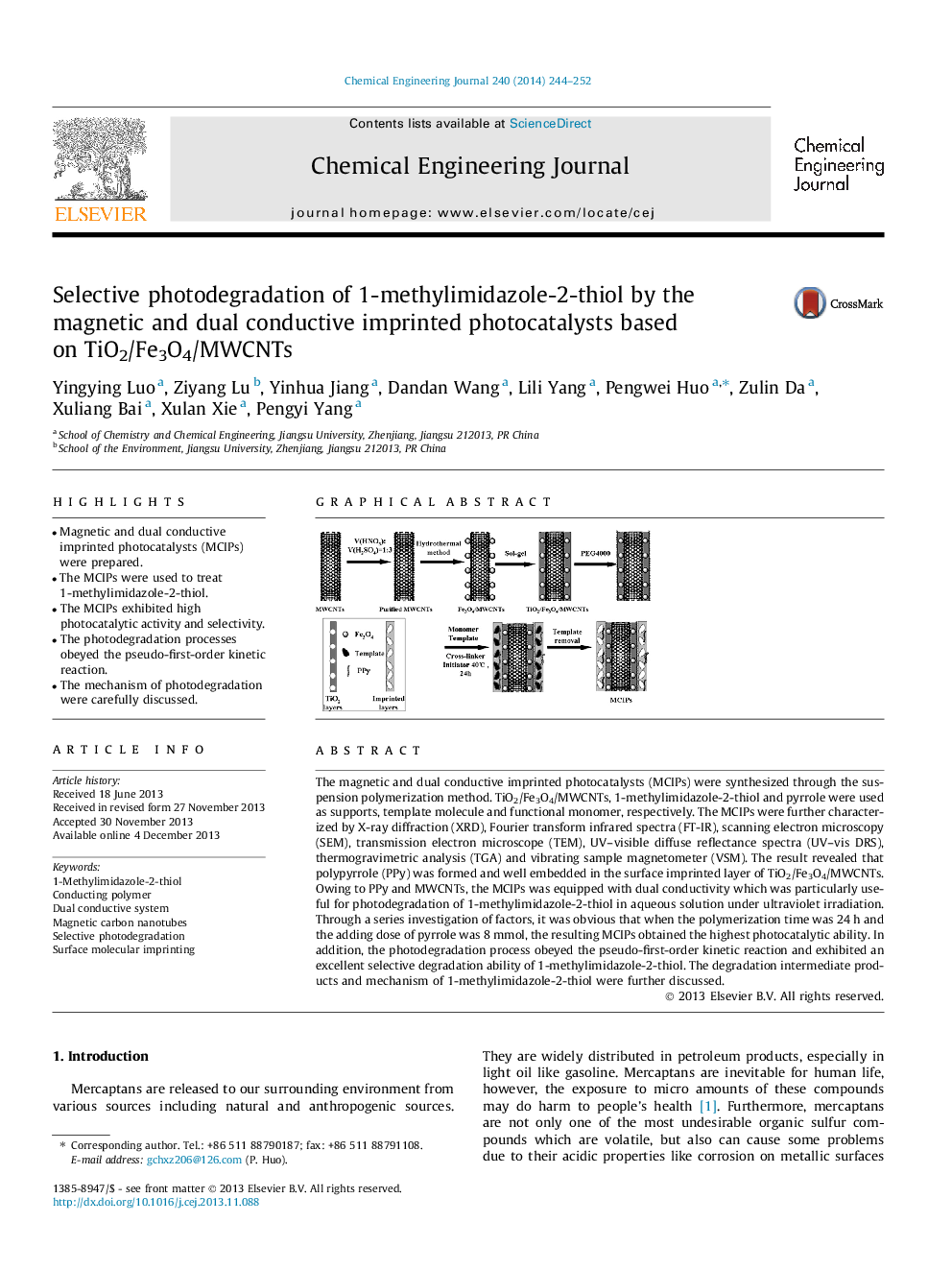
The magnetic and dual conductive imprinted photocatalysts (MCIPs) were synthesized through the suspension polymerization method. TiO2/Fe3O4/MWCNTs, 1-methylimidazole-2-thiol and pyrrole were used as supports, template molecule and functional monomer, respectively. The MCIPs were further characterized by X-ray diffraction (XRD), Fourier transform infrared spectra (FT-IR), scanning electron microscopy (SEM), transmission electron microscope (TEM), UV–visible diffuse reflectance spectra (UV–vis DRS), thermogravimetric analysis (TGA) and vibrating sample magnetometer (VSM). The result revealed that polypyrrole (PPy) was formed and well embedded in the surface imprinted layer of TiO2/Fe3O4/MWCNTs. Owing to PPy and MWCNTs, the MCIPs was equipped with dual conductivity which was particularly useful for photodegradation of 1-methylimidazole-2-thiol in aqueous solution under ultraviolet irradiation. Through a series investigation of factors, it was obvious that when the polymerization time was 24 h and the adding dose of pyrrole was 8 mmol, the resulting MCIPs obtained the highest photocatalytic ability. In addition, the photodegradation process obeyed the pseudo-first-order kinetic reaction and exhibited an excellent selective degradation ability of 1-methylimidazole-2-thiol. The degradation intermediate products and mechanism of 1-methylimidazole-2-thiol were further discussed.
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 240, 15 March 2014, Pages 244–252