| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 148696 | 456421 | 2013 | 9 صفحه PDF | دانلود رایگان |
• Study on the adsorption of dimethyl sulfide (DMS) from liquid hydrocarbon streams.
• It demonstrated a high saturated sulfur capacity of 80 mg S/g adsorbent.
• Combined techniques of in situ FTIR and DMS-TPD were used.
• Adsorption mechanism of DMS on adsorbents was investigated.
A high-performance adsorbent was developed by loading silver on bentonite for the adsorption of dimethyl sulfide (DMS) from liquid hydrocarbon streams under ambient conditions. It demonstrated a high saturated sulfur capacity of 80 mg S/g adsorbent when the bentonite was loaded with 8 wt% Ag+ and calcined at 150 °C. The prepared adsorbents were characterized by adsorption of nitrogen (BET), Transmission Electron Microscopy (TEM), X-ray diffraction (XRD), pyridine Fourier transform infrared spectroscopy (pyridine-FTIR) and Thermogravimetric Analysis (TG). The results showed that the silver species dispersed on the surface of bentonite had an important role in determining the DMS removal performance while the surface acid sites of adsorbent had little correlation with the DMS removal performance. Combined techniques of temperature programmed desorption of DMS (DMS-TPD) and in situ Fourier transform infrared spectroscopy (in situ FTIR) were used to investigate the adsorption mechanism of DMS on raw bentonite and Ag+ modified bentonite, respectively. The results revealed that three interaction patterns were existed between the adsorbed DMS and raw bentonite while two interaction patterns were observed between adsorbed DMS and Ag+ modified bentonite. The DMS molecules hardly entered into the interlayer of Ag+ modified bentonite due to the strong interactions between DMS molecules and silver cations.
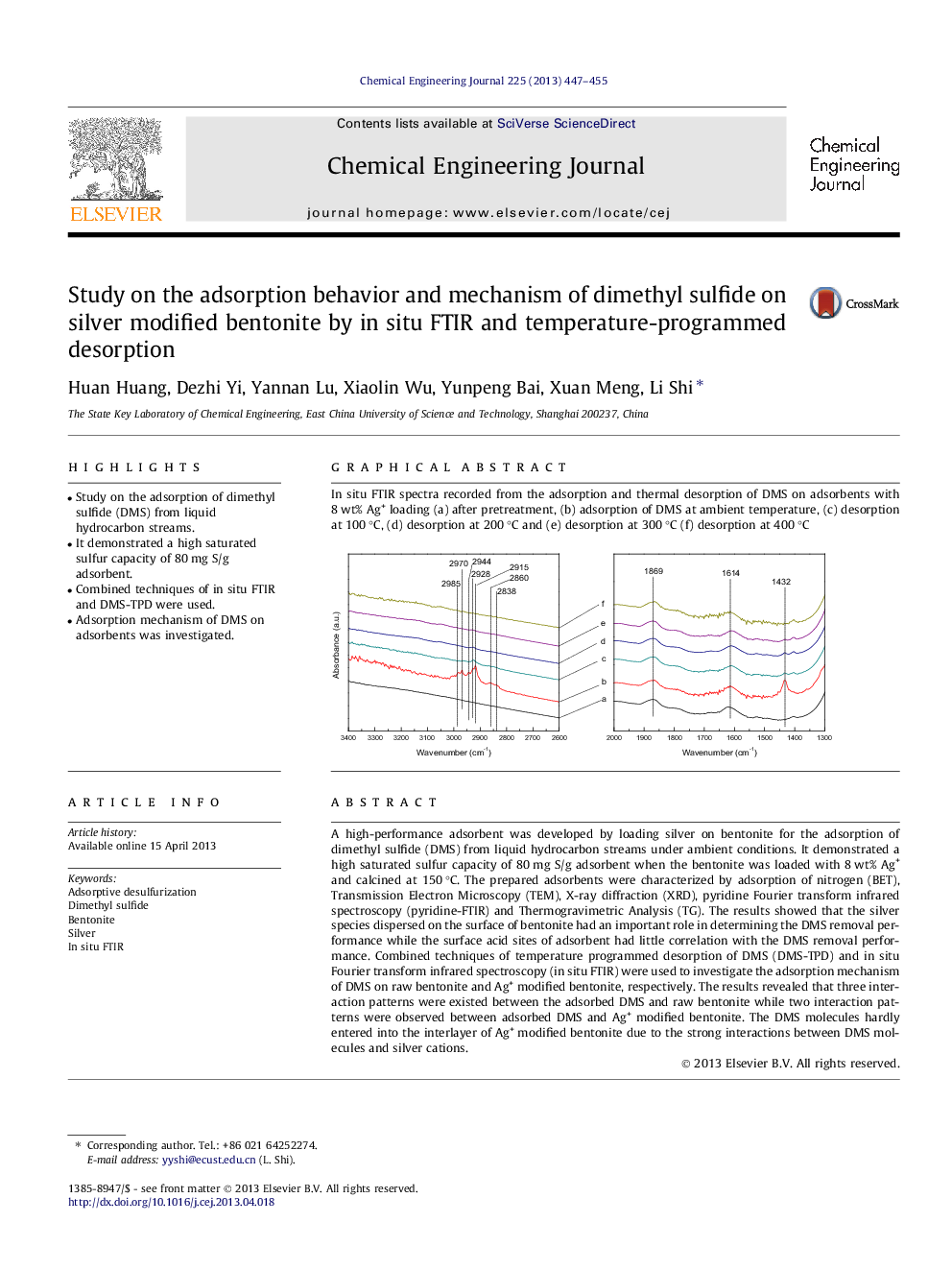
In situ FTIR spectra recorded from the adsorption and thermal desorption of DMS on adsorbents with 8 wt% Ag+ loading (a) after pretreatment, (b) adsorption of DMS at ambient temperature, (c) desorption at 100 °C, (d) desorption at 200 °C and (e) desorption at 300 °C (f) desorption at 400 °CFigure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 225, 1 June 2013, Pages 447–455