| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 148782 | 456422 | 2013 | 9 صفحه PDF | دانلود رایگان |
Adsorptive removal of 2-naphthol from aqueous solutions by a hypercrosslinked poly(styrene-co-divinylbenzene) (PS) resin (TEPA-3) was investigated under adsorption equilibrium, kinetic and column breakthrough conditions. The adsorption of 2-naphthol in aqueous solutions on resin TEPA-3 is very effective as indicated by the high adsorption equilibrium capacity (210.0 mg/g at 2-naphthol concentration of 100 mg/L and 298 K). This adsorption process is endothermic because the adsorption capacity increases with the feed solution temperature. The adsorption of 2-naphthol in aqueous solutions on resin TEPA-3 could reach equilibrium within 180 min, the adsorption kinetic data can be well correlated by the pseudo-second-order rate equation and a micropore diffusion model, and the diffusion activation energy was determined to be 4.998 kJ/mol. The dynamic adsorption capacities obtained in the column breakthrough experiments are within 90% of the corresponding equilibrium capacities obtained in the batch experiments. The Bed Depth Service Time model, the Thomas and Yoon model are suitable for fitting the column breakthrough data. The used TEPA-3 resin saturated with 2-naphthol can be effectively regenerated with a mixture of 1% of sodium hydroxide (w/v) and 50% of ethanol aqueous solution (v/v).
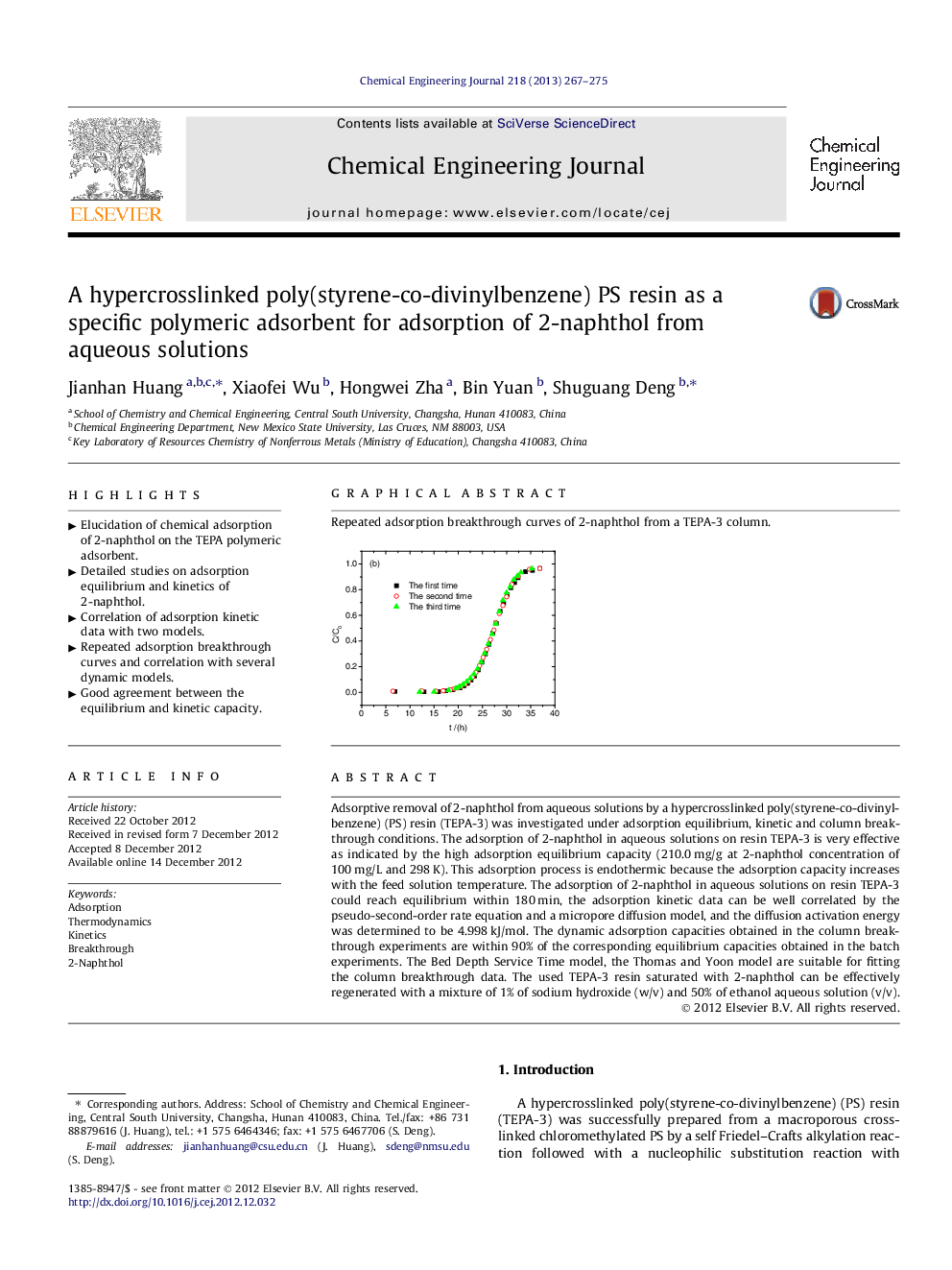
Repeated adsorption breakthrough curves of 2-naphthol from a TEPA-3 column.Figure optionsDownload as PowerPoint slideHighlights
• Elucidation of chemical adsorption of 2-naphthol on the TEPA polymeric adsorbent.
• Detailed studies on adsorption equilibrium and kinetics of 2-naphthol.
• Correlation of adsorption kinetic data with two models.
• Repeated adsorption breakthrough curves and correlation with several dynamic models.
• Good agreement between the equilibrium and kinetic capacity.
Journal: Chemical Engineering Journal - Volume 218, 15 February 2013, Pages 267–275