| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 149200 | 456429 | 2012 | 8 صفحه PDF | دانلود رایگان |
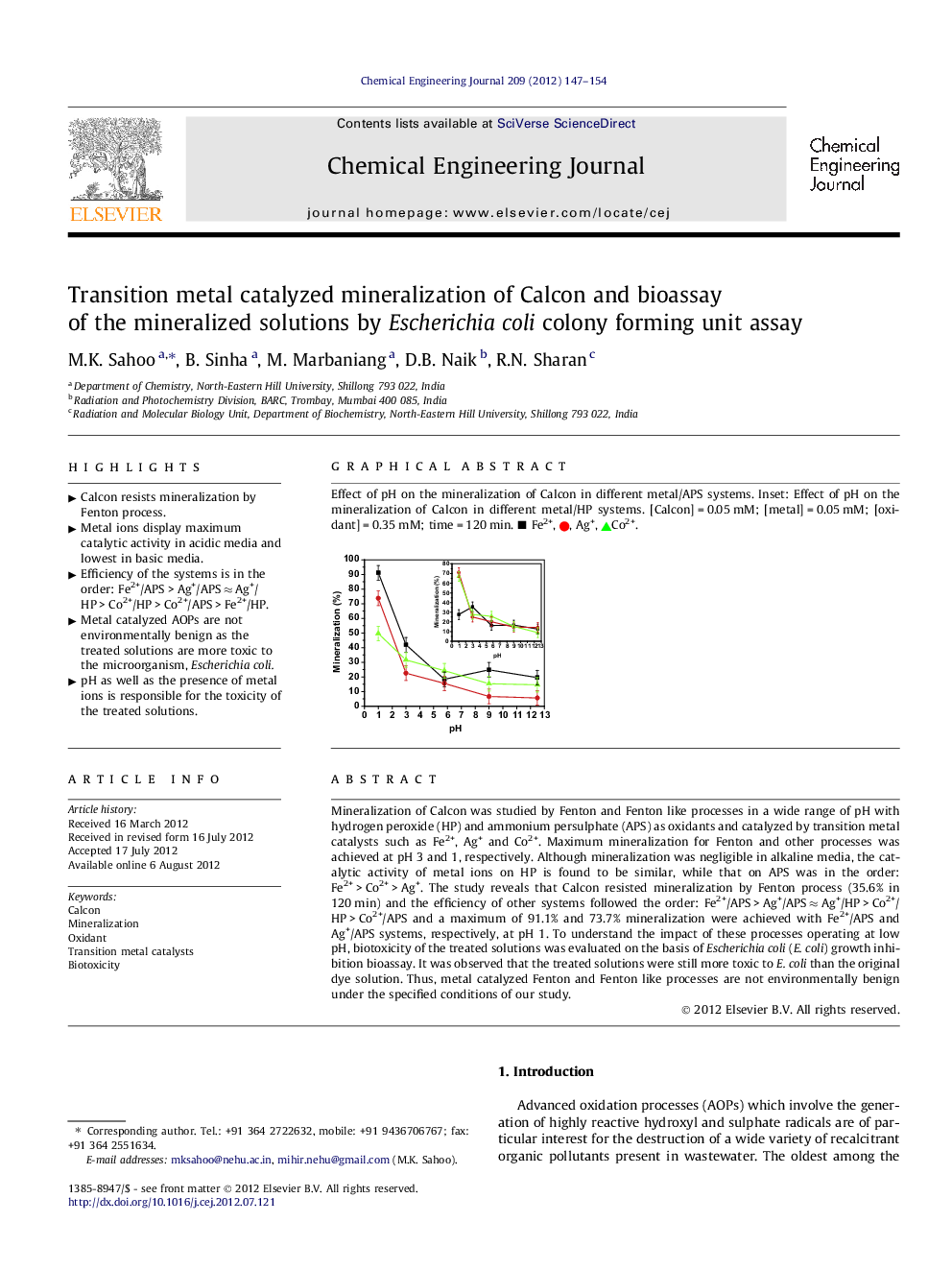
Mineralization of Calcon was studied by Fenton and Fenton like processes in a wide range of pH with hydrogen peroxide (HP) and ammonium persulphate (APS) as oxidants and catalyzed by transition metal catalysts such as Fe2+, Ag+ and Co2+. Maximum mineralization for Fenton and other processes was achieved at pH 3 and 1, respectively. Although mineralization was negligible in alkaline media, the catalytic activity of metal ions on HP is found to be similar, while that on APS was in the order: Fe2+ > Co2+ > Ag+. The study reveals that Calcon resisted mineralization by Fenton process (35.6% in 120 min) and the efficiency of other systems followed the order: Fe2+/APS > Ag+/APS ≈ Ag+/HP > Co2+/HP > Co2+/APS and a maximum of 91.1% and 73.7% mineralization were achieved with Fe2+/APS and Ag+/APS systems, respectively, at pH 1. To understand the impact of these processes operating at low pH, biotoxicity of the treated solutions was evaluated on the basis of Escherichia coli (E. coli) growth inhibition bioassay. It was observed that the treated solutions were still more toxic to E. coli than the original dye solution. Thus, metal catalyzed Fenton and Fenton like processes are not environmentally benign under the specified conditions of our study.
Effect of pH on the mineralization of Calcon in different metal/APS systems. Inset: Effect of pH on the mineralization of Calcon in different metal/HP systems. [Calcon] = 0.05 mM; [metal] = 0.05 mM; [oxidant] = 0.35 mM; time = 120 min. ■ Fe2+, , Ag+, Co2+.Figure optionsDownload as PowerPoint slideFigure optionsDownload as PowerPoint slideFigure optionsDownload as PowerPoint slideHighlights
► Calcon resists mineralization by Fenton process.
► Metal ions display maximum catalytic activity in acidic media and lowest in basic media.
► Efficiency of the systems is in the order: Fe2+/APS > Ag+/APS ≈ Ag+/HP > Co2+/HP > Co2+/APS > Fe2+/HP.
► Metal catalyzed AOPs are not environmentally benign as the treated solutions are more toxic to the microorganism, Escherichia coli.
► pH as well as the presence of metal ions is responsible for the toxicity of the treated solutions.
Journal: Chemical Engineering Journal - Volume 209, 15 October 2012, Pages 147–154