| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 149673 | 456435 | 2012 | 5 صفحه PDF | دانلود رایگان |
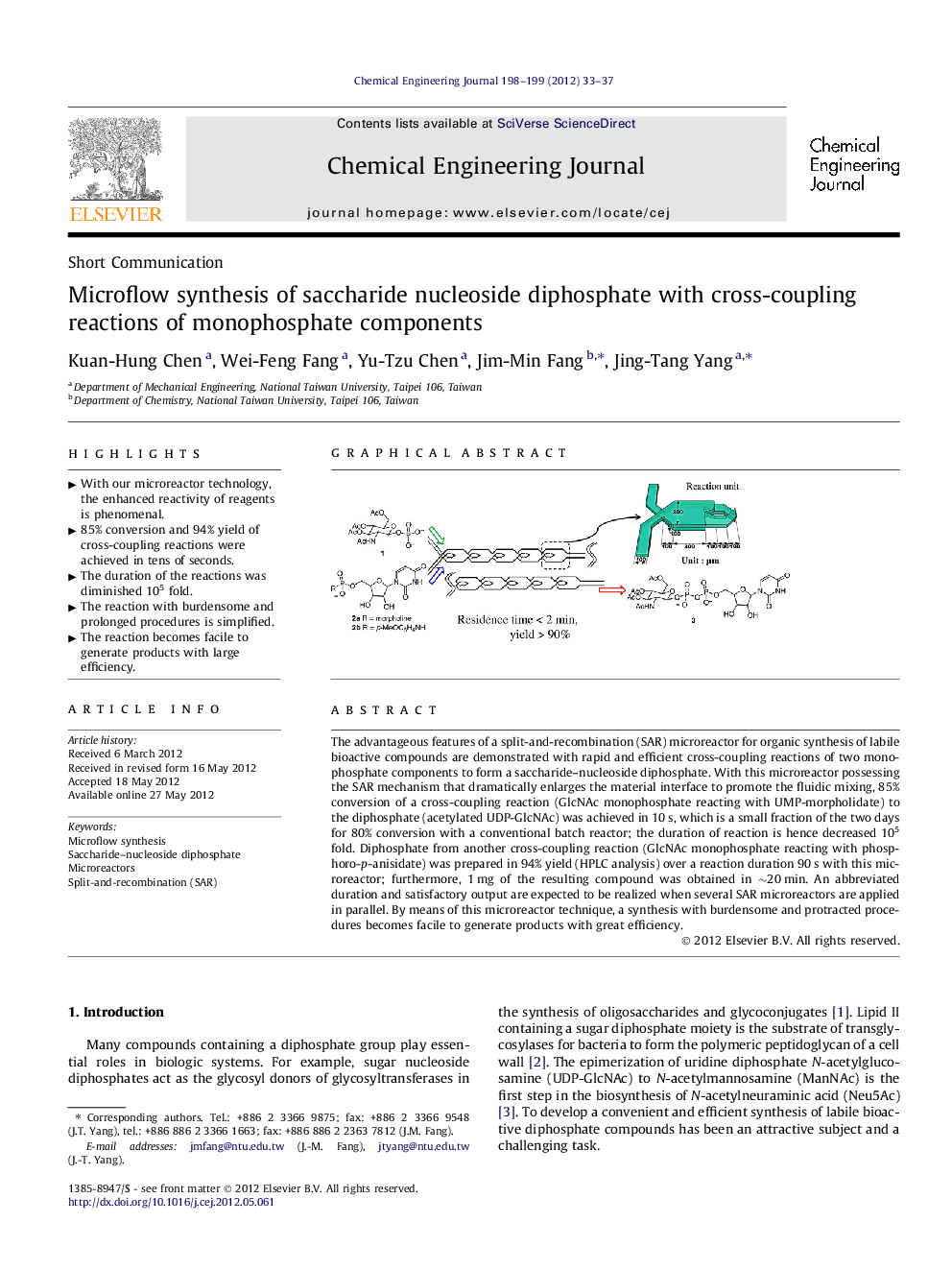
The advantageous features of a split-and-recombination (SAR) microreactor for organic synthesis of labile bioactive compounds are demonstrated with rapid and efficient cross-coupling reactions of two monophosphate components to form a saccharide–nucleoside diphosphate. With this microreactor possessing the SAR mechanism that dramatically enlarges the material interface to promote the fluidic mixing, 85% conversion of a cross-coupling reaction (GlcNAc monophosphate reacting with UMP-morpholidate) to the diphosphate (acetylated UDP-GlcNAc) was achieved in 10 s, which is a small fraction of the two days for 80% conversion with a conventional batch reactor; the duration of reaction is hence decreased 105 fold. Diphosphate from another cross-coupling reaction (GlcNAc monophosphate reacting with phosphoro-p-anisidate) was prepared in 94% yield (HPLC analysis) over a reaction duration 90 s with this microreactor; furthermore, 1 mg of the resulting compound was obtained in ∼20 min. An abbreviated duration and satisfactory output are expected to be realized when several SAR microreactors are applied in parallel. By means of this microreactor technique, a synthesis with burdensome and protracted procedures becomes facile to generate products with great efficiency.
Figure optionsDownload as PowerPoint slideHighlights
► With our microreactor technology, the enhanced reactivity of reagents is phenomenal.
► 85% conversion and 94% yield of cross-coupling reactions were achieved in tens of seconds.
► The duration of the reactions was diminished 105 fold.
► The reaction with burdensome and prolonged procedures is simplified.
► The reaction becomes facile to generate products with large efficiency.
Journal: Chemical Engineering Journal - Volumes 198–199, 1 August 2012, Pages 33–37