| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 149765 | 456437 | 2012 | 8 صفحه PDF | دانلود رایگان |
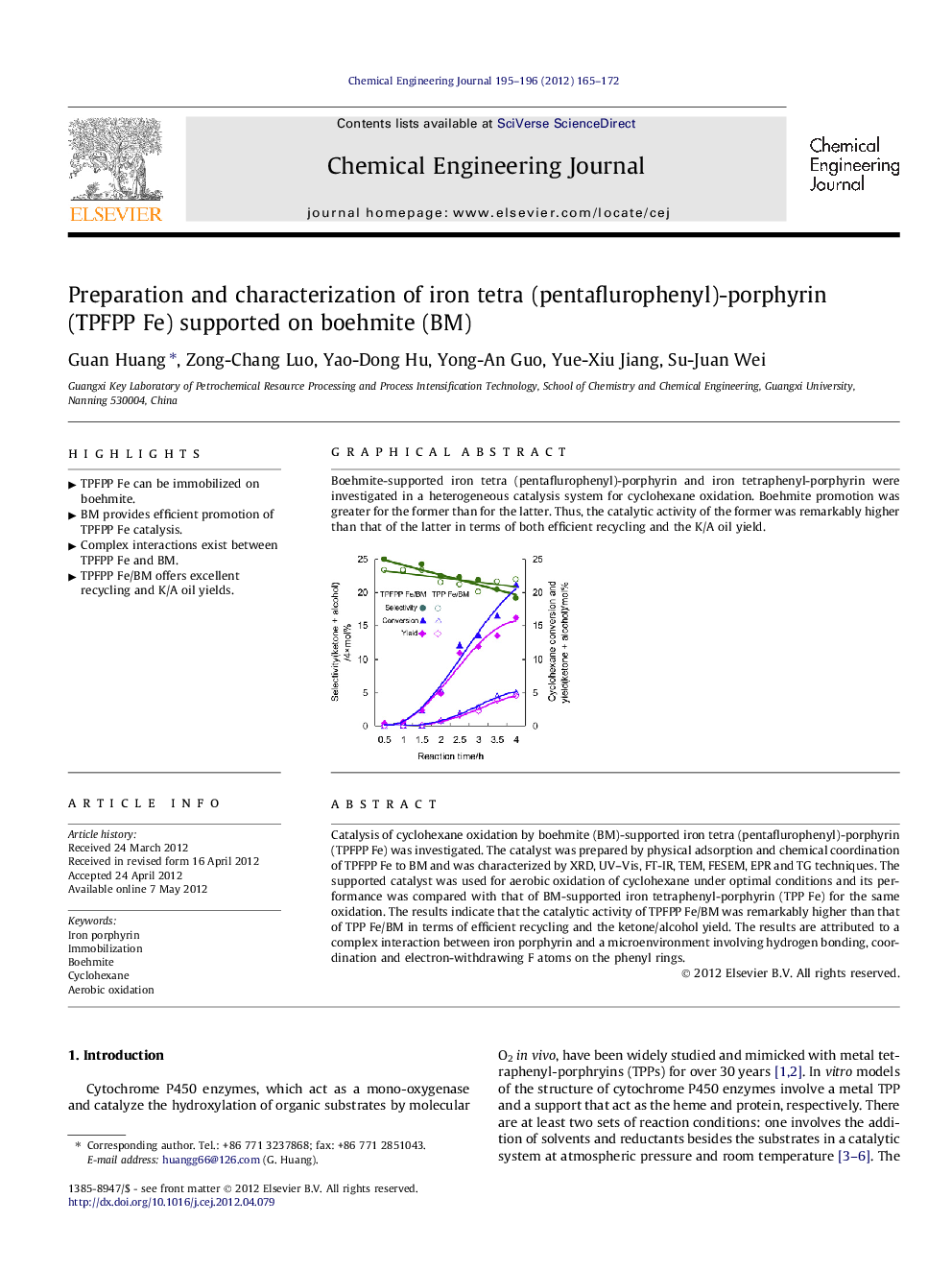
Catalysis of cyclohexane oxidation by boehmite (BM)-supported iron tetra (pentaflurophenyl)-porphyrin (TPFPP Fe) was investigated. The catalyst was prepared by physical adsorption and chemical coordination of TPFPP Fe to BM and was characterized by XRD, UV–Vis, FT-IR, TEM, FESEM, EPR and TG techniques. The supported catalyst was used for aerobic oxidation of cyclohexane under optimal conditions and its performance was compared with that of BM-supported iron tetraphenyl-porphyrin (TPP Fe) for the same oxidation. The results indicate that the catalytic activity of TPFPP Fe/BM was remarkably higher than that of TPP Fe/BM in terms of efficient recycling and the ketone/alcohol yield. The results are attributed to a complex interaction between iron porphyrin and a microenvironment involving hydrogen bonding, coordination and electron-withdrawing F atoms on the phenyl rings.
Boehmite-supported iron tetra (pentaflurophenyl)-porphyrin and iron tetraphenyl-porphyrin were investigated in a heterogeneous catalysis system for cyclohexane oxidation. Boehmite promotion was greater for the former than for the latter. Thus, the catalytic activity of the former was remarkably higher than that of the latter in terms of both efficient recycling and the K/A oil yield.Figure optionsDownload as PowerPoint slideHighlights
► TPFPP Fe can be immobilized on boehmite.
► BM provides efficient promotion of TPFPP Fe catalysis.
► Complex interactions exist between TPFPP Fe and BM.
► TPFPP Fe/BM offers excellent recycling and K/A oil yields.
Journal: Chemical Engineering Journal - Volumes 195–196, 1 July 2012, Pages 165–172