| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1606217 | 1516220 | 2016 | 7 صفحه PDF | دانلود رایگان |
عنوان انگلیسی مقاله ISI
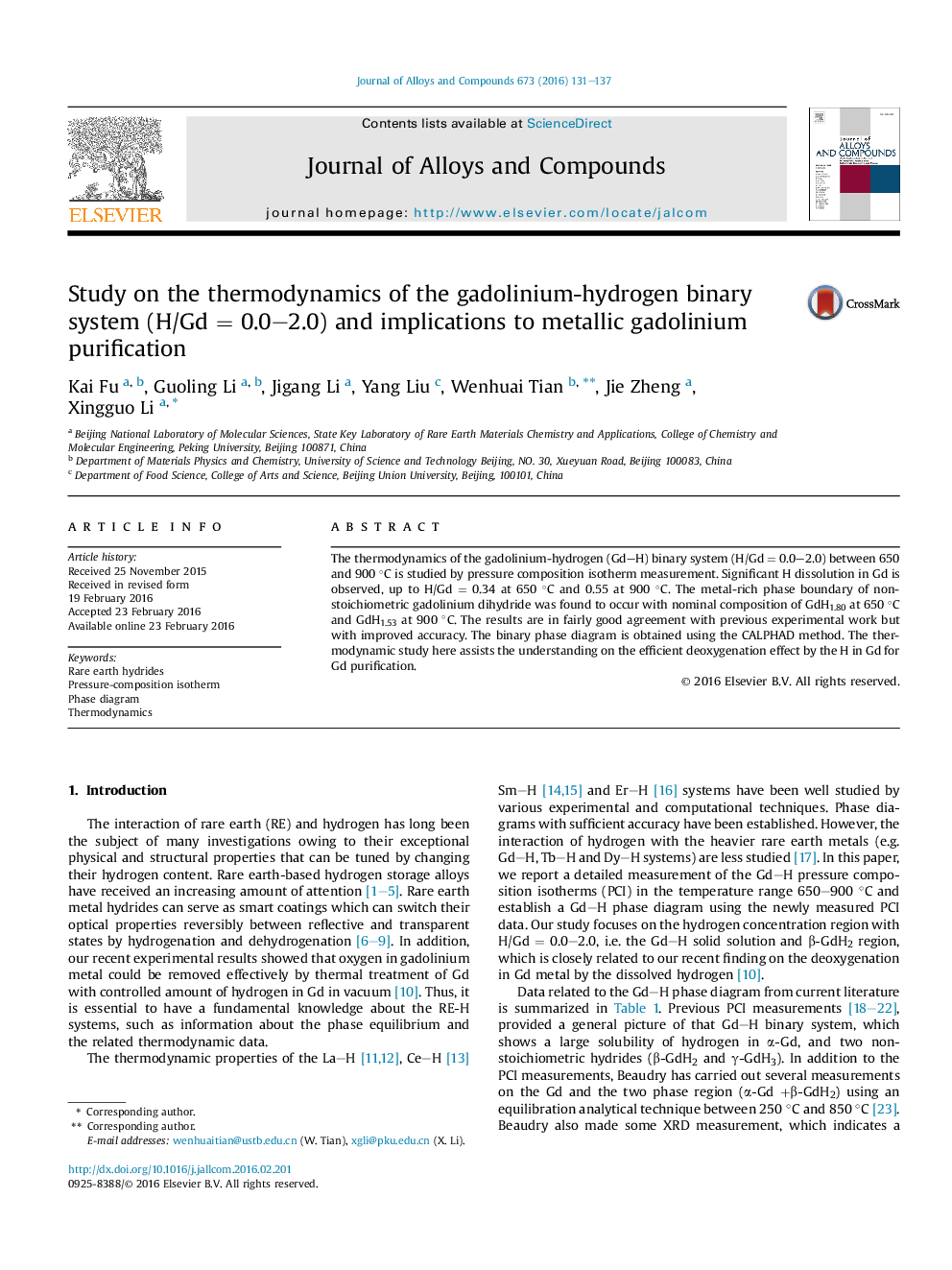
Study on the thermodynamics of the gadolinium-hydrogen binary system (H/Gd = 0.0-2.0) and implications to metallic gadolinium purification
دانلود مقاله + سفارش ترجمه
دانلود مقاله ISI انگلیسی
رایگان برای ایرانیان
کلمات کلیدی
موضوعات مرتبط
مهندسی و علوم پایه
مهندسی مواد
فلزات و آلیاژها
پیش نمایش صفحه اول مقاله
چکیده انگلیسی
The thermodynamics of the gadolinium-hydrogen (Gd-H) binary system (H/Gd = 0.0-2.0) between 650 and 900 °C is studied by pressure composition isotherm measurement. Significant H dissolution in Gd is observed, up to H/Gd = 0.34 at 650 °C and 0.55 at 900 °C. The metal-rich phase boundary of nonstoichiometric gadolinium dihydride was found to occur with nominal composition of GdH1.80 at 650 °C and GdH1.53 at 900 °C. The results are in fairly good agreement with previous experimental work but with improved accuracy. The binary phase diagram is obtained using the CALPHAD method. The thermodynamic study here assists the understanding on the efficient deoxygenation effect by the H in Gd for Gd purification.
ناشر
Database: Elsevier - ScienceDirect (ساینس دایرکت)
Journal: Journal of Alloys and Compounds - Volume 673, 15 July 2016, Pages 131-137
Journal: Journal of Alloys and Compounds - Volume 673, 15 July 2016, Pages 131-137
نویسندگان
Kai Fu, Guoling Li, Jigang Li, Yang Liu, Wenhuai Tian, Jie Zheng, Xingguo Li,