| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1654323 | 1007690 | 2006 | 5 صفحه PDF | دانلود رایگان |
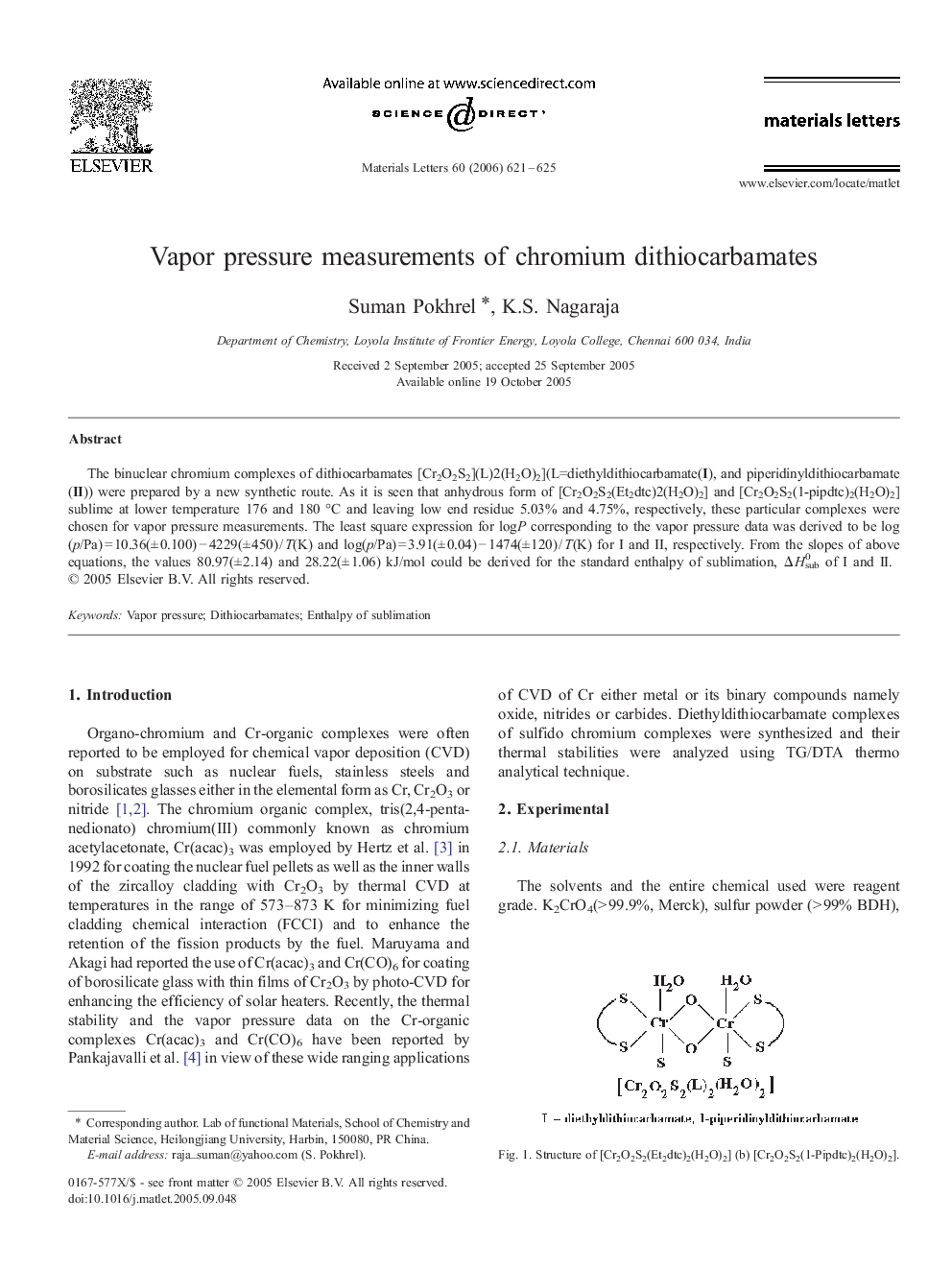
The binuclear chromium complexes of dithiocarbamates [Cr2O2S2](L)2(H2O)2](L=diethyldithiocarbamate(I), and piperidinyldithiocarbamate(II)) were prepared by a new synthetic route. As it is seen that anhydrous form of [Cr2O2S2(Et2dtc)2(H2O)2] and [Cr2O2S2(1-pipdtc)2(H2O)2] sublime at lower temperature 176 and 180 °C and leaving low end residue 5.03% and 4.75%, respectively, these particular complexes were chosen for vapor pressure measurements. The least square expression for logP corresponding to the vapor pressure data was derived to be log(p/Pa) = 10.36(± 0.100) − 4229(± 450) / T(K) and log(p/Pa) = 3.91(± 0.04) − 1474(± 120) / T(K) for I and II, respectively. From the slopes of above equations, the values 80.97(± 2.14) and 28.22(± 1.06) kJ/mol could be derived for the standard enthalpy of sublimation, ΔHsub0 of I and II.
Journal: Materials Letters - Volume 60, Issue 5, March 2006, Pages 621–625