| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 4407926 | 1618825 | 2016 | 11 صفحه PDF | دانلود رایگان |
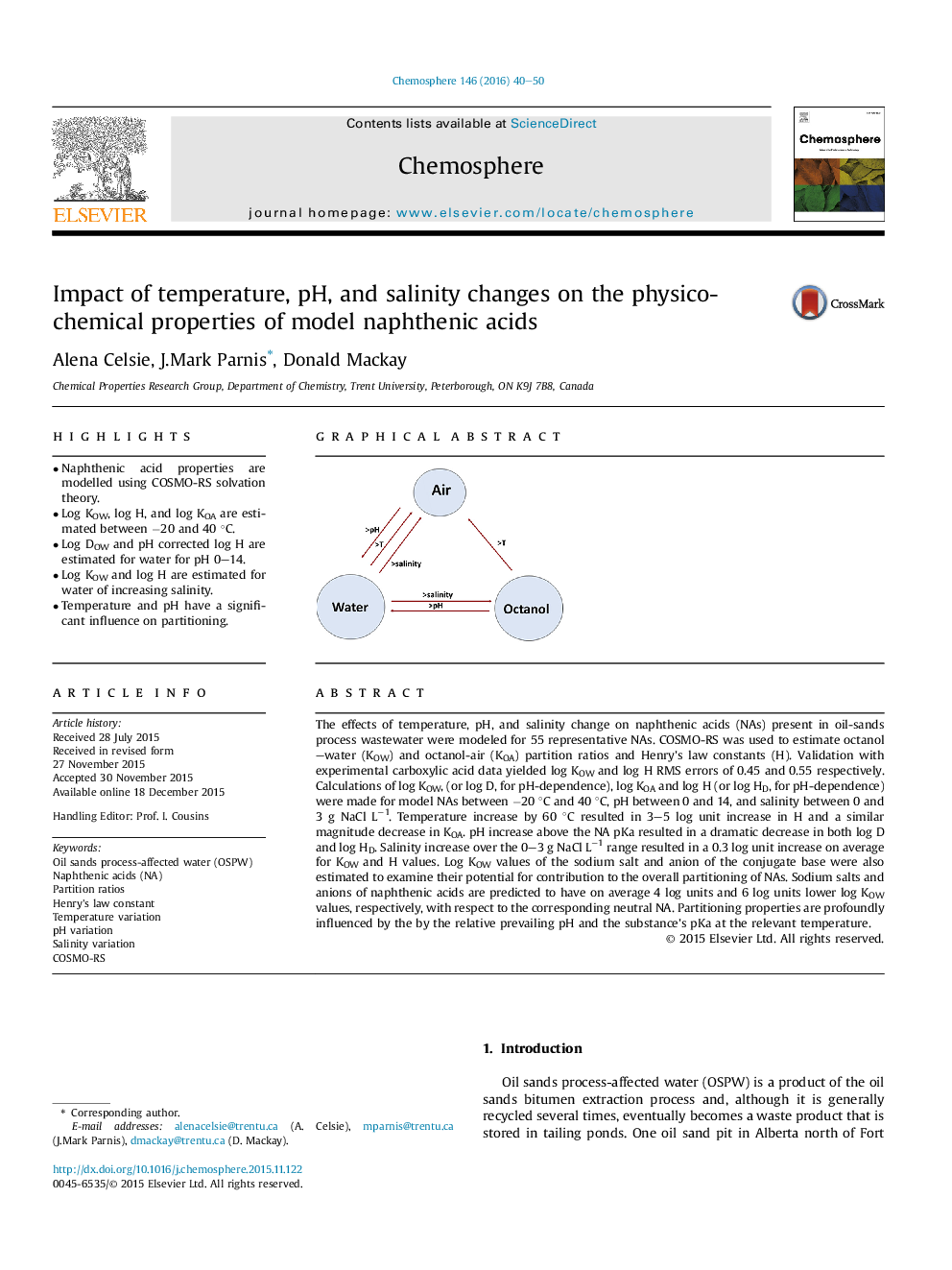
• Naphthenic acid properties are modelled using COSMO-RS solvation theory.
• Log KOW, log H, and log KOA are estimated between −20 and 40 °C.
• Log DOW and pH corrected log H are estimated for water for pH 0–14.
• Log KOW and log H are estimated for water of increasing salinity.
• Temperature and pH have a significant influence on partitioning.
The effects of temperature, pH, and salinity change on naphthenic acids (NAs) present in oil-sands process wastewater were modeled for 55 representative NAs. COSMO-RS was used to estimate octanol–water (KOW) and octanol-air (KOA) partition ratios and Henry's law constants (H). Validation with experimental carboxylic acid data yielded log KOW and log H RMS errors of 0.45 and 0.55 respectively. Calculations of log KOW, (or log D, for pH-dependence), log KOA and log H (or log HD, for pH-dependence) were made for model NAs between −20 °C and 40 °C, pH between 0 and 14, and salinity between 0 and 3 g NaCl L−1. Temperature increase by 60 °C resulted in 3–5 log unit increase in H and a similar magnitude decrease in KOA. pH increase above the NA pKa resulted in a dramatic decrease in both log D and log HD. Salinity increase over the 0–3 g NaCl L−1 range resulted in a 0.3 log unit increase on average for KOW and H values. Log KOW values of the sodium salt and anion of the conjugate base were also estimated to examine their potential for contribution to the overall partitioning of NAs. Sodium salts and anions of naphthenic acids are predicted to have on average 4 log units and 6 log units lower log KOW values, respectively, with respect to the corresponding neutral NA. Partitioning properties are profoundly influenced by the by the relative prevailing pH and the substance's pKa at the relevant temperature.
Figure optionsDownload as PowerPoint slide
Journal: Chemosphere - Volume 146, March 2016, Pages 40–50