| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 4408760 | 1618858 | 2014 | 7 صفحه PDF | دانلود رایگان |
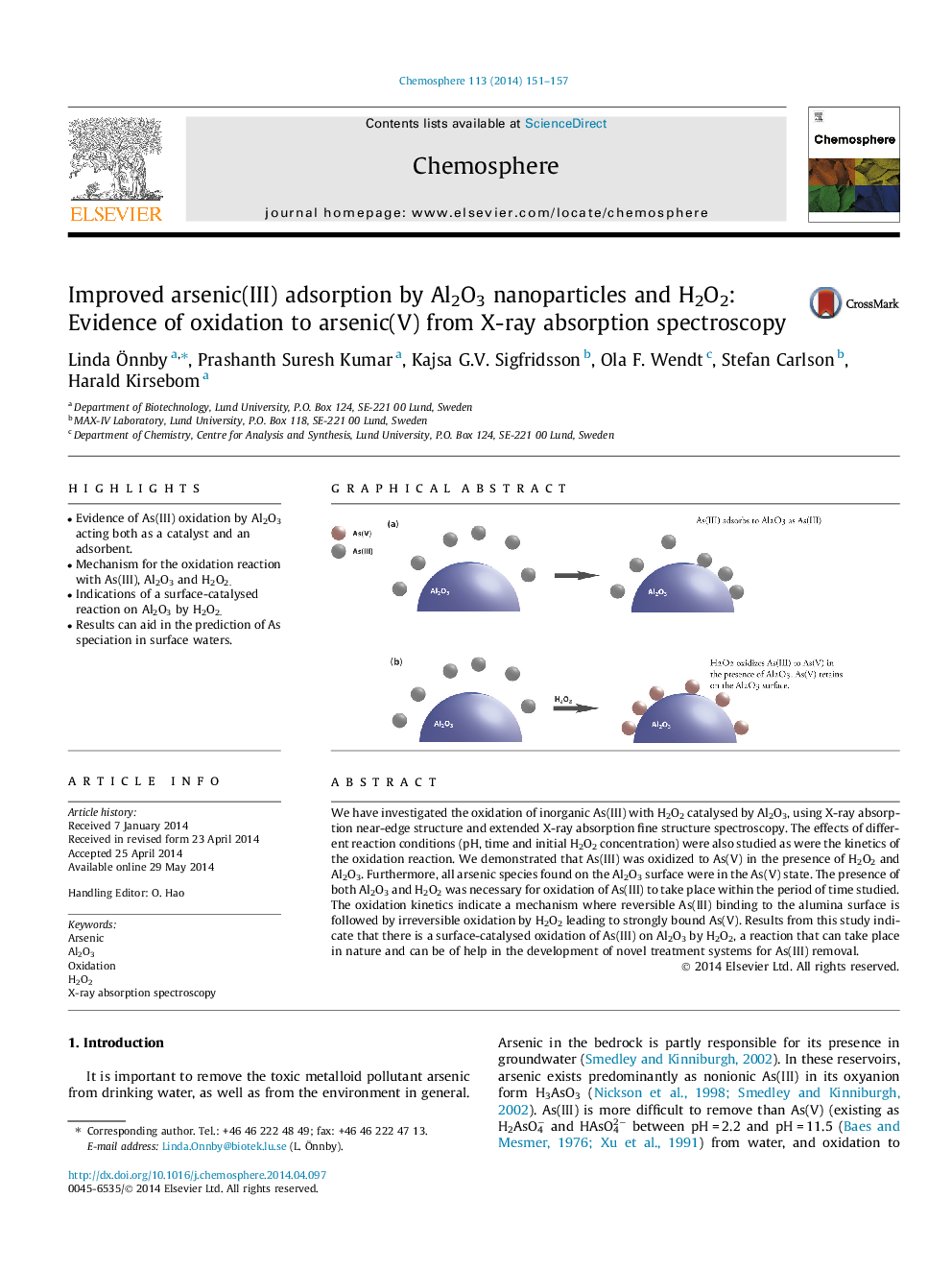
• Evidence of As(III) oxidation by Al2O3 acting both as a catalyst and an adsorbent.
• Mechanism for the oxidation reaction with As(III), Al2O3 and H2O2.
• Indications of a surface-catalysed reaction on Al2O3 by H2O2.
• Results can aid in the prediction of As speciation in surface waters.
We have investigated the oxidation of inorganic As(III) with H2O2 catalysed by Al2O3, using X-ray absorption near-edge structure and extended X-ray absorption fine structure spectroscopy. The effects of different reaction conditions (pH, time and initial H2O2 concentration) were also studied as were the kinetics of the oxidation reaction. We demonstrated that As(III) was oxidized to As(V) in the presence of H2O2 and Al2O3. Furthermore, all arsenic species found on the Al2O3 surface were in the As(V) state. The presence of both Al2O3 and H2O2 was necessary for oxidation of As(III) to take place within the period of time studied. The oxidation kinetics indicate a mechanism where reversible As(III) binding to the alumina surface is followed by irreversible oxidation by H2O2 leading to strongly bound As(V). Results from this study indicate that there is a surface-catalysed oxidation of As(III) on Al2O3 by H2O2, a reaction that can take place in nature and can be of help in the development of novel treatment systems for As(III) removal.
Figure optionsDownload as PowerPoint slide
Journal: Chemosphere - Volume 113, October 2014, Pages 151–157