| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 4751866 | 1642727 | 2017 | 10 صفحه PDF | دانلود رایگان |
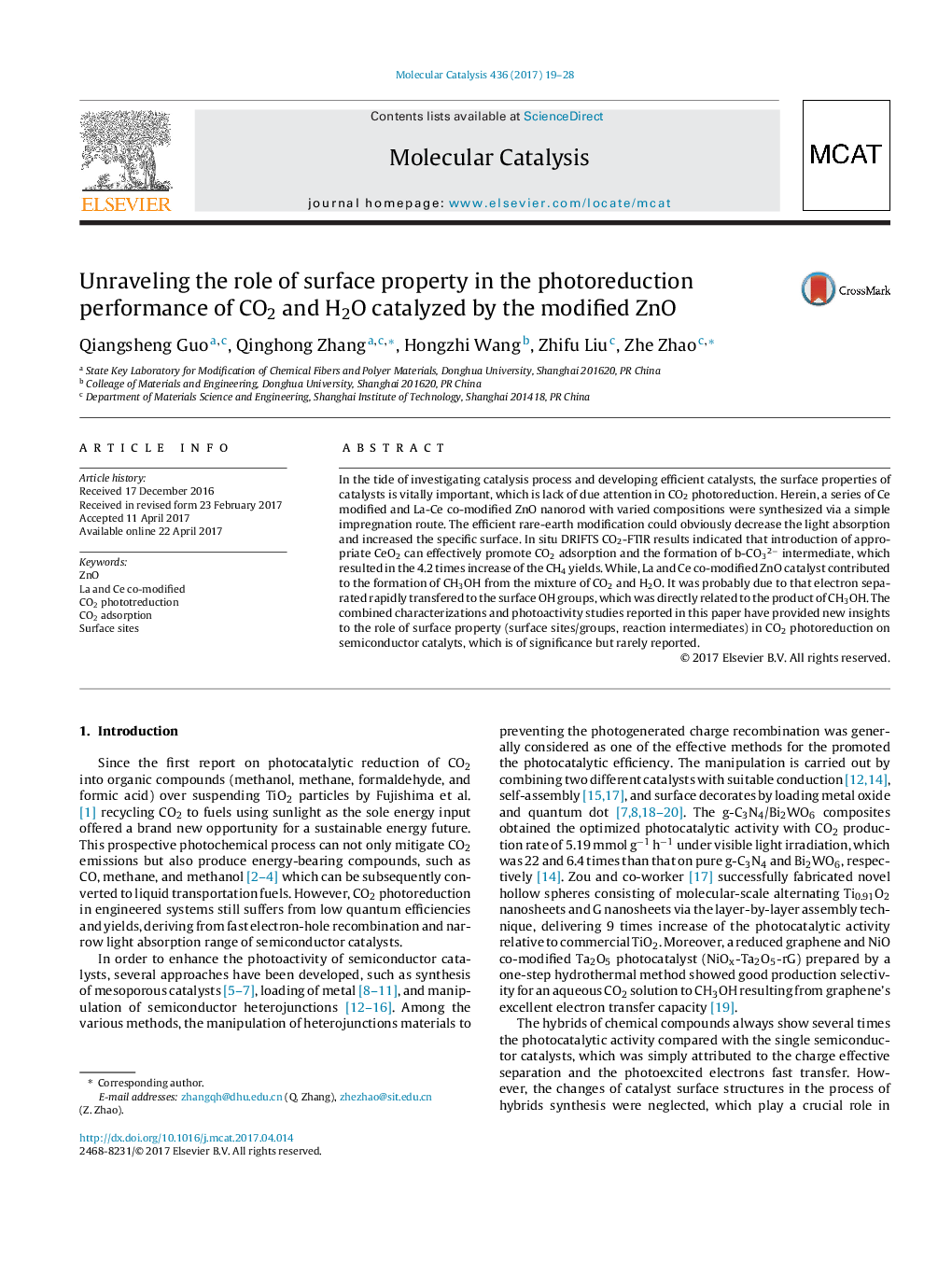
- The efficient rare-earth modification of ZnO could obviously enhance the CH4 and CH3OH yields due to significant increase of CO2 adsorption.
- The introduction of CeO2 species to ZnO can significantly promote the selectivity and yield of CH4, while the La and Ce co-modified ZnO effectively increases the catalytic process of CO2 to CH3OH.
- The observably increased b-CO3 2â intermediates after Ce modified, which are possible intermediates for the production of CH4.
- La and Ce co-modified ZnO catalyst contributed to the formation of CH3OH, which was probably due to that electron separated rapidly transferred to HCO3 - intermediates (directly related to the product of CH3OH).
- CO2 molecular react with two active sites, forming b-CO32â, HCO3â intermediates and produce to CH4 and CH3OH after the process of C-O bond breaking and C-H bond formation, respectively
In the tide of investigating catalysis process and developing efficient catalysts, the surface properties of catalysts is vitally important, which is lack of due attention in CO2 photoreduction. Herein, a series of Ce modified and La-Ce co-modified ZnO nanorod with varied compositions were synthesized via a simple impregnation route. The efficient rare-earth modification could obviously decrease the light absorption and increased the specific surface. In situ DRIFTS CO2-FTIR results indicated that introduction of appropriate CeO2 can effectively promote CO2 adsorption and the formation of b-CO32â intermediate, which resulted in the 4.2 times increase of the CH4 yields. While, La and Ce co-modified ZnO catalyst contributed to the formation of CH3OH from the mixture of CO2 and H2O. It was probably due to that electron separated rapidly transfered to the surface OH groups, which was directly related to the product of CH3OH. The combined characterizations and photoactivity studies reported in this paper have provided new insights to the role of surface property (surface sites/groups, reaction intermediates) in CO2 photoreduction on semiconductor catalyts, which is of significance but rarely reported.
Me+-Oâ centers or oxygen vacancies are the active sites of CH4 formation, and surface OH groups are closely linked with CH3OH synthesis from CO2 photoreduction. CO2 molecular react with two active sites, forming b-CO32â, HCO3- intermediates and produce to CH4 and CH3OH after the process of CO bond breaking and CH bond formation, respectively.
Journal: Molecular Catalysis - Volume 436, July 2017, Pages 19-28