| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 4762790 | 1422947 | 2018 | 8 صفحه PDF | دانلود رایگان |
- Distinguishing adsorption zones by using adsorption acceleration graphs.
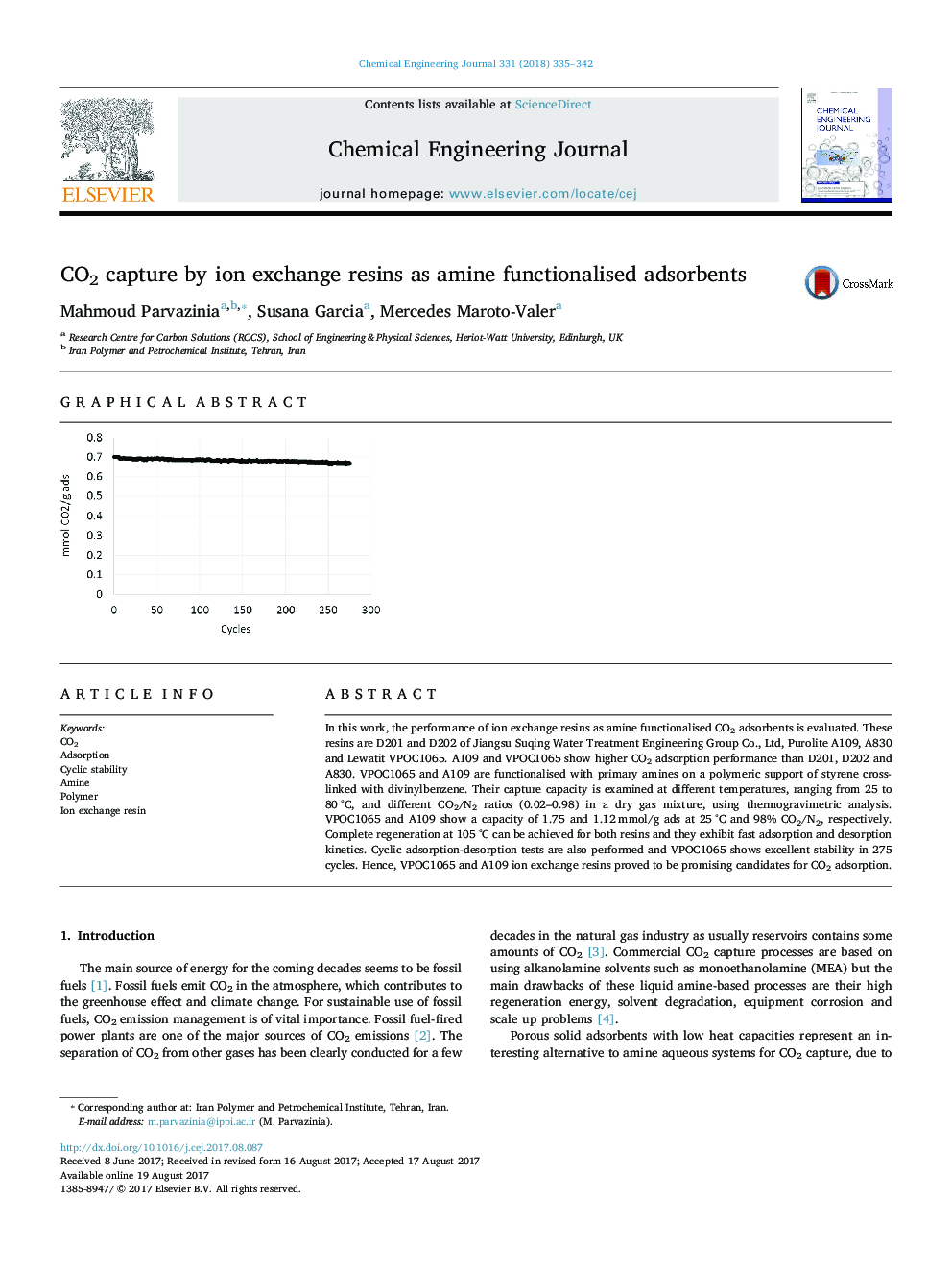
- Excellent cyclic adsorption-desorption stability after 275 cycles for VPOC1065.
- Fast and complete CO2 desorption at 105 °C based on desorption graphs and IR absorption data for both A109 and VPOC1065.
In this work, the performance of ion exchange resins as amine functionalised CO2 adsorbents is evaluated. These resins are D201 and D202 of Jiangsu Suqing Water Treatment Engineering Group Co., Ltd, Purolite A109, A830 and Lewatit VPOC1065. A109 and VPOC1065 show higher CO2 adsorption performance than D201, D202 and A830. VPOC1065 and A109 are functionalised with primary amines on a polymeric support of styrene cross-linked with divinylbenzene. Their capture capacity is examined at different temperatures, ranging from 25 to 80 °C, and different CO2/N2 ratios (0.02-0.98) in a dry gas mixture, using thermogravimetric analysis. VPOC1065 and A109 show a capacity of 1.75 and 1.12 mmol/g ads at 25 °C and 98% CO2/N2, respectively. Complete regeneration at 105 °C can be achieved for both resins and they exhibit fast adsorption and desorption kinetics. Cyclic adsorption-desorption tests are also performed and VPOC1065 shows excellent stability in 275 cycles. Hence, VPOC1065 and A109 ion exchange resins proved to be promising candidates for CO2 adsorption.
28
Journal: Chemical Engineering Journal - Volume 331, 1 January 2018, Pages 335-342