| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5219673 | 1383364 | 2012 | 8 صفحه PDF | دانلود رایگان |
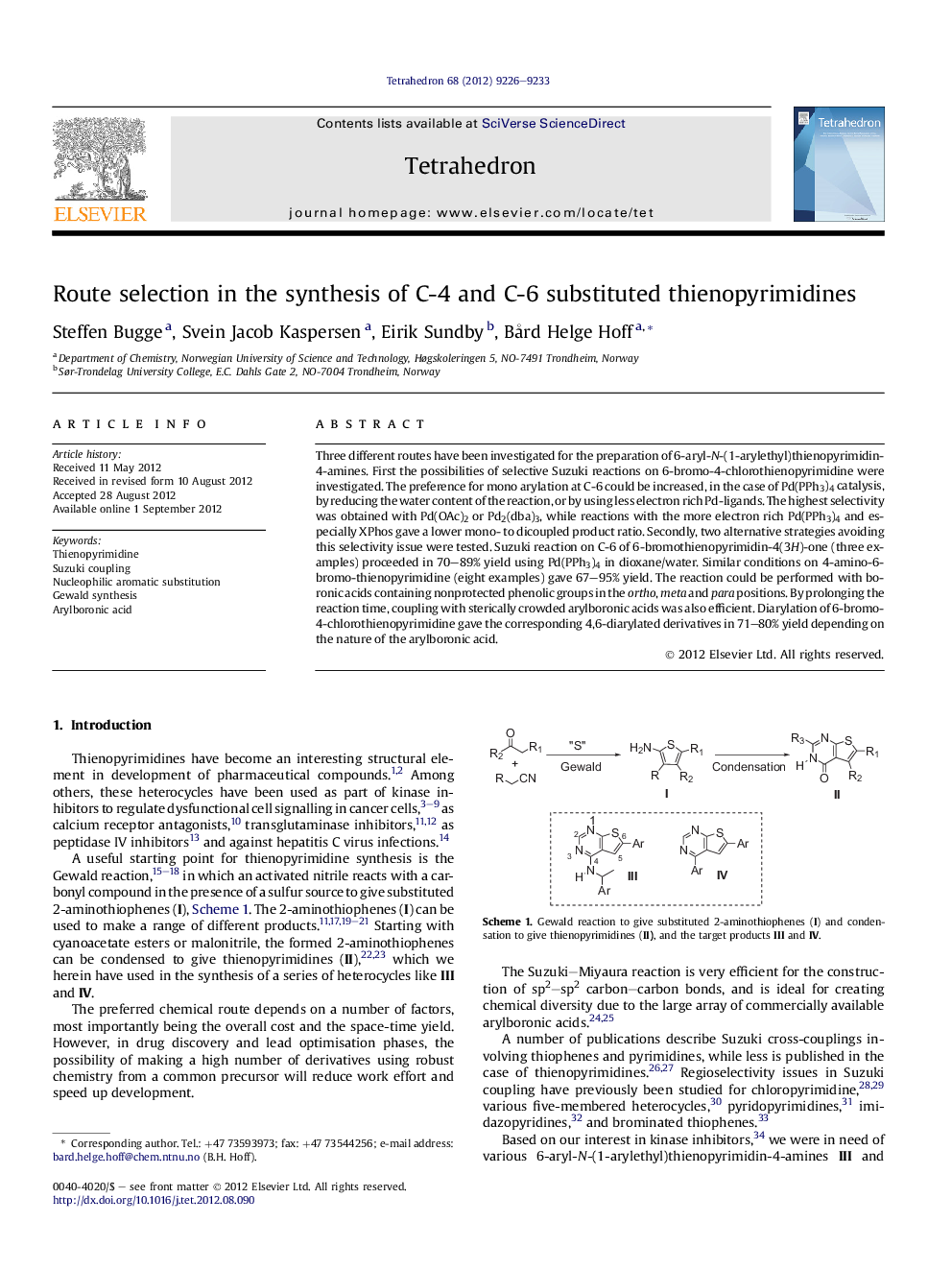
Three different routes have been investigated for the preparation of 6-aryl-N-(1-arylethyl)thienopyrimidin-4-amines. First the possibilities of selective Suzuki reactions on 6-bromo-4-chlorothienopyrimidine were investigated. The preference for mono arylation at C-6 could be increased, in the case of Pd(PPh3)4 catalysis, by reducing the water content of the reaction, or by using less electron rich Pd-ligands. The highest selectivity was obtained with Pd(OAc)2 or Pd2(dba)3, while reactions with the more electron rich Pd(PPh3)4 and especially XPhos gave a lower mono- to dicoupled product ratio. Secondly, two alternative strategies avoiding this selectivity issue were tested. Suzuki reaction on C-6 of 6-bromothienopyrimidin-4(3H)-one (three examples) proceeded in 70-89% yield using Pd(PPh3)4 in dioxane/water. Similar conditions on 4-amino-6-bromo-thienopyrimidine (eight examples) gave 67-95% yield. The reaction could be performed with boronic acids containing nonprotected phenolic groups in the ortho, meta and para positions. By prolonging the reaction time, coupling with sterically crowded arylboronic acids was also efficient. Diarylation of 6-bromo-4-chlorothienopyrimidine gave the corresponding 4,6-diarylated derivatives in 71-80% yield depending on the nature of the arylboronic acid.
Journal: Tetrahedron - Volume 68, Issue 45, 11 November 2012, Pages 9226-9233