| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5221336 | 1383417 | 2012 | 8 صفحه PDF | دانلود رایگان |
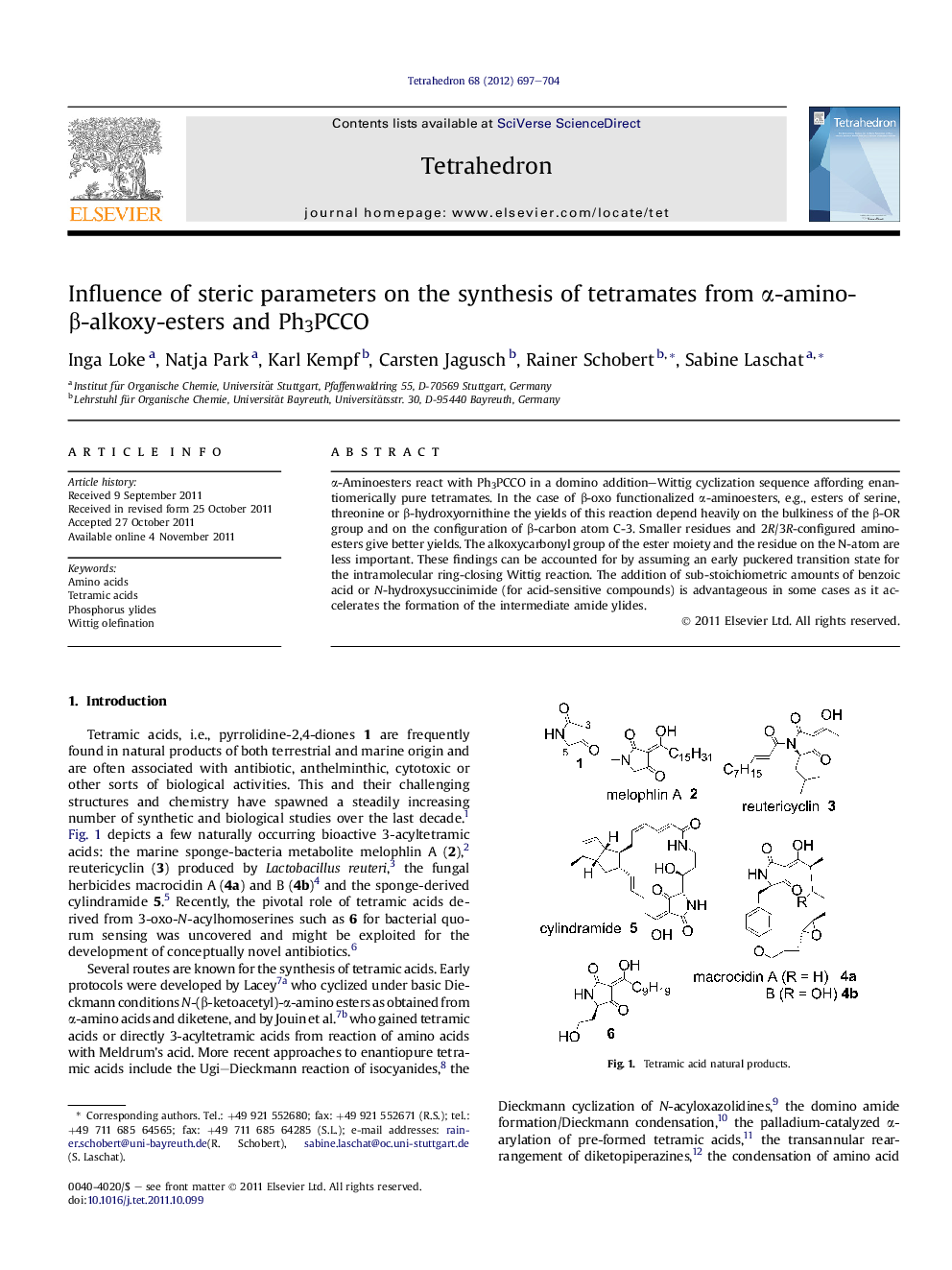
α-Aminoesters react with Ph3PCCO in a domino addition-Wittig cyclization sequence affording enantiomerically pure tetramates. In the case of β-oxo functionalized α-aminoesters, e.g., esters of serine, threonine or β-hydroxyornithine the yields of this reaction depend heavily on the bulkiness of the β-OR group and on the configuration of β-carbon atom C-3. Smaller residues and 2R/3R-configured aminoesters give better yields. The alkoxycarbonyl group of the ester moiety and the residue on the N-atom are less important. These findings can be accounted for by assuming an early puckered transition state for the intramolecular ring-closing Wittig reaction. The addition of sub-stoichiometric amounts of benzoic acid or N-hydroxysuccinimide (for acid-sensitive compounds) is advantageous in some cases as it accelerates the formation of the intermediate amide ylides.
Journal: Tetrahedron - Volume 68, Issue 2, 14 January 2012, Pages 697-704