| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5223986 | 1383504 | 2010 | 11 صفحه PDF | دانلود رایگان |
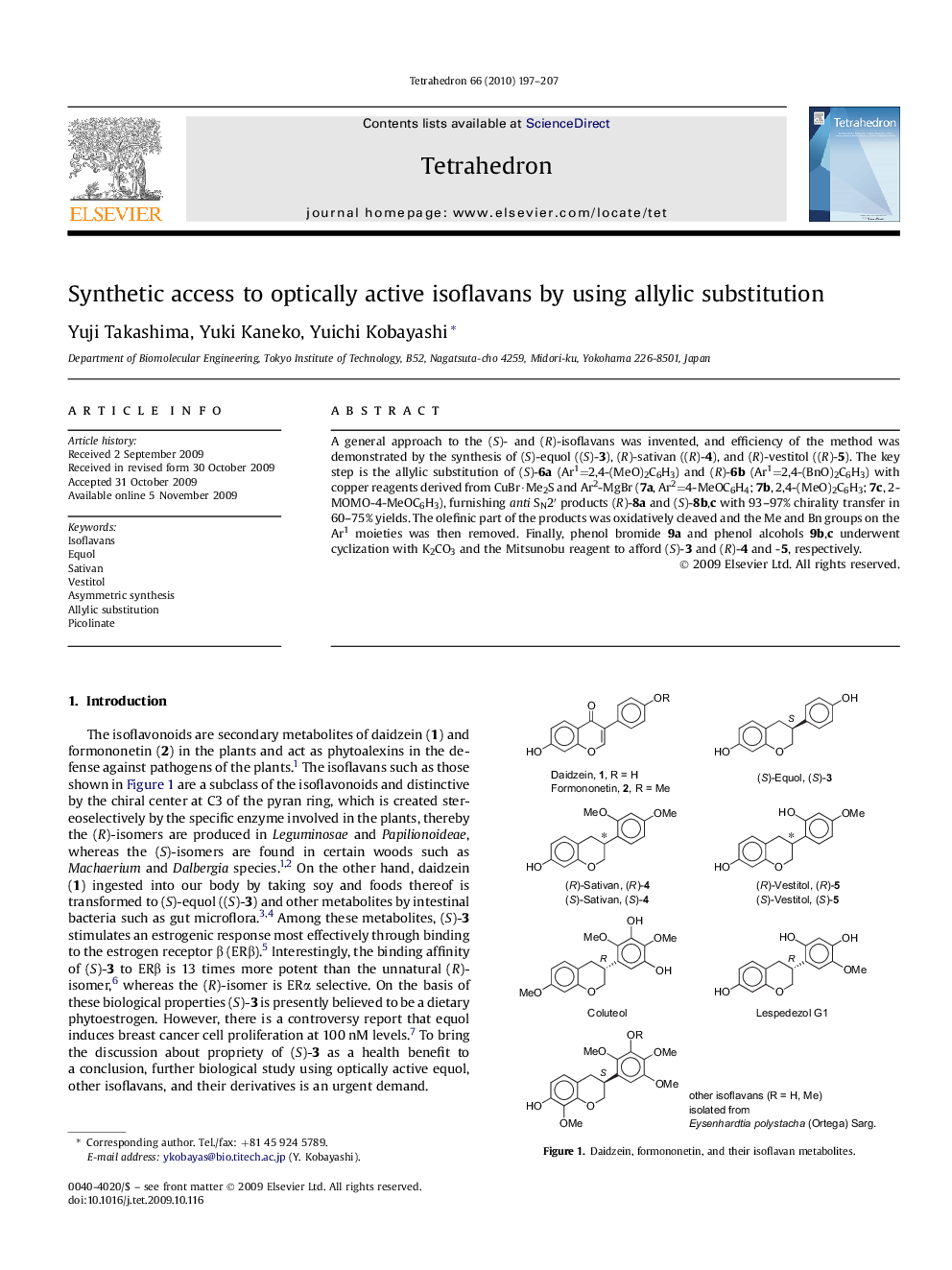
A general approach to the (S)- and (R)-isoflavans was invented, and efficiency of the method was demonstrated by the synthesis of (S)-equol ((S)-3), (R)-sativan ((R)-4), and (R)-vestitol ((R)-5). The key step is the allylic substitution of (S)-6a (Ar1=2,4-(MeO)2C6H3) and (R)-6b (Ar1=2,4-(BnO)2C6H3) with copper reagents derived from CuBr·Me2S and Ar2-MgBr (7a, Ar2=4-MeOC6H4; 7b, 2,4-(MeO)2C6H3; 7c, 2-MOMO-4-MeOC6H3), furnishing anti SN2Ⲡproducts (R)-8a and (S)-8b,c with 93-97% chirality transfer in 60-75% yields. The olefinic part of the products was oxidatively cleaved and the Me and Bn groups on the Ar1 moieties was then removed. Finally, phenol bromide 9a and phenol alcohols 9b,c underwent cyclization with K2CO3 and the Mitsunobu reagent to afford (S)-3 and (R)-4 and -5, respectively.
Journal: Tetrahedron - Volume 66, Issue 1, 2 January 2010, Pages 197-207