| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5370800 | 1503912 | 2015 | 8 صفحه PDF | دانلود رایگان |
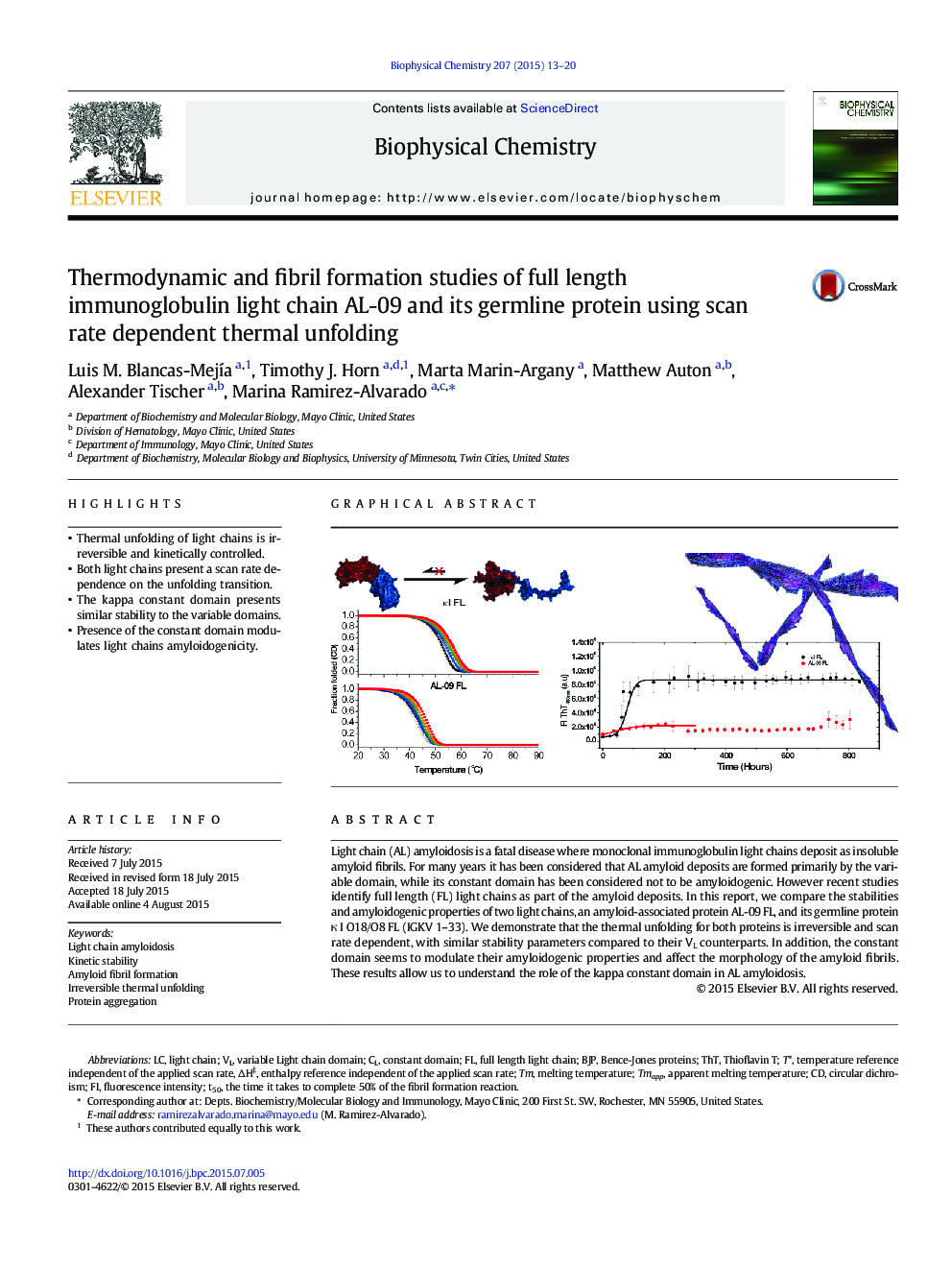
- Thermal unfolding of light chains is irreversible and kinetically controlled.
- Both light chains present a scan rate dependence on the unfolding transition.
- The kappa constant domain presents similar stability to the variable domains.
- Presence of the constant domain modulates light chains amyloidogenicity.
Light chain (AL) amyloidosis is a fatal disease where monoclonal immunoglobulin light chains deposit as insoluble amyloid fibrils. For many years it has been considered that AL amyloid deposits are formed primarily by the variable domain, while its constant domain has been considered not to be amyloidogenic. However recent studies identify full length (FL) light chains as part of the amyloid deposits. In this report, we compare the stabilities and amyloidogenic properties of two light chains, an amyloid-associated protein AL-09 FL, and its germline protein κ I O18/O8 FL (IGKV 1-33). We demonstrate that the thermal unfolding for both proteins is irreversible and scan rate dependent, with similar stability parameters compared to their VL counterparts. In addition, the constant domain seems to modulate their amyloidogenic properties and affect the morphology of the amyloid fibrils. These results allow us to understand the role of the kappa constant domain in AL amyloidosis.
Journal: Biophysical Chemistry - Volume 207, December 2015, Pages 13-20